Peer Reviewed
Newly Diagnosed Chronic Myeloid Leukemia With Leukostasis
AUTHORS:
Arine Musaelyan, MD1 • Katrina Au, MD1 • Xinlai Sun, MD2 • Stefan Balan, MD3
AFFILIATIONS:
1Internal Medicine Department, Jersey City Medical Center, Jersey City, New Jersey
2Director and Chair, Department of Pathology and Laboratory Medicine, Jersey City Medical Center, Rutgers University New Jersey Medical School, Jersey City, New Jersey
3Chief, Oncology Department, Jersey City Medical Center, Jersey City, New Jersey
CITATION:
Musaelyan A, Au K, Sun X, Balan S. Newly diagnosed chronic myeloid leukemia with leukostasis. Consultant. 2022;62(7):e17-e19. doi:10.25270/con.2021.12.00005
Received March 28, 2021. Accepted May 17, 2021. Published online December 20, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Arine Musaelyan, MD, Jersey City Medical Center, 355 Grand Street, Jersey City, NJ, 07302 (arine.md@gmail.com)
A 25-year-old woman presented to our urgent care center with a 3-day history of dizziness, a 3-month history of increasing abdominal fullness, a 2-week history of drenching night sweats, and new-onset of emesis and blurry vision on the day of presentation.
She denied any easy bruising or bleeding. Her medical and family histories were noncontributory.
Physical examination. Upon evaluation at the urgent care center, the patient was found to have hypoxia with an oxygen saturation of 80% on room air. She was immediately sent to the emergency department, where she remained persistently hypoxic in the 80% range while on a nasal cannula. She was afebrile, normotensive with a blood pressure of 100/67 mm Hg, and a heart rate of 100 beats/min.
The physical examination was significant for splenomegaly, with the spleen crossing the midline and extending to the pelvic rim. Her arterial blood gas level was examined, which was consistent with acute hypoxic respiratory failure with a partial pressure of oxygen (pO2) of 41.2 mm Hg on room air.
Diagnostic testing. The results were significant for leukocytosis with a white blood cell count of 700 K/UL, anemia with a hemoglobin level of 9.1 g/dL, and thrombocytosis with a platelet count of 636 K/UL. The manual differential revealed increased granulocytes in different stages of maturation with left shift. A peripheral smear was obtained, which demonstrated numerous myeloid cells at different early stages (Figure). Moreover, the patient had an elevated uric acid level of 8.9 mg/dL, lactate dehydrogenase level of 3571 U/L, a normal D-dimer level of 0.45 ug/mL, and a normal fibrinogen level of 304 mg/dL. Results of chest radiography and electrocardiography scans were unremarkable.
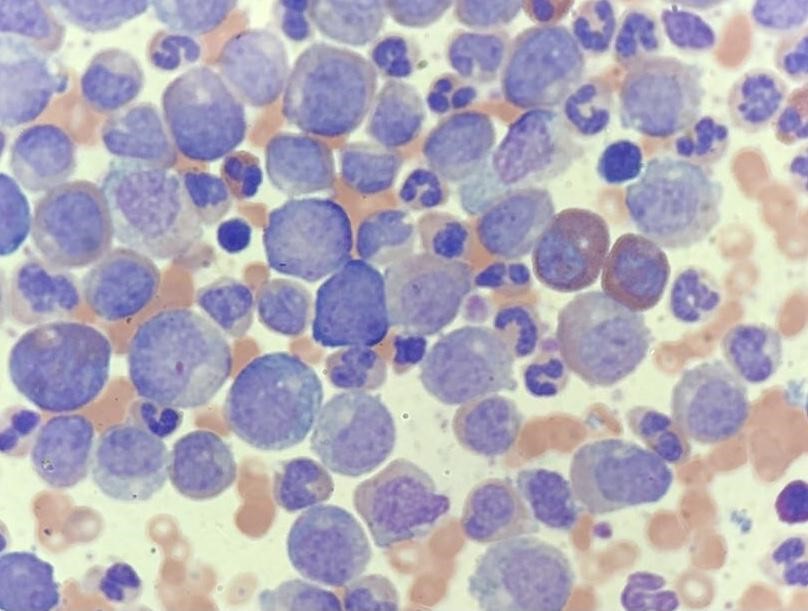
A peripheral blood polymerase chain reaction test for BCR/ABL major p210 fusion protein resulted in 27.17% on the international scale, thus establishing the diagnosis of chronic myeloid leukemia (CML). By the peripheral blood flow cytometry analysis, CD34 positive blasts accounted for 0.8% of the total events. The myeloid cells compromised approximately 88% of the total events and expressed CD33, CD13, CD11b, subset CD16, subset CD38, subset CD10, and aberrant expression of CD56. Additionally, results of fluorescence in situ hybridization (FISH) studies were positive for t(9,22).
Treatment and management. The patient underwent leukapheresis, was initiated on oral hydroxyurea, 3 g, 3 times daily for debulking of her CML, and was initiated on imatinib, 400 mg daily. The patient’s respiratory status improved over her 10-day hospital stay, with her oxygen saturation stable at 95% on room air. Upon discharge, imatinib was changed to dasatinib, 100 mg daily—a second-generation tyrosine-kinase inhibitor that has shown to offer better disease control.
At her 1-month follow-up, the patient’s symptoms had improved with dasatinib treatment. The patient reported an improvement in dizziness, a reduction in abdominal fullness, and normalized vision. She continued to report moderate fatigue. Her spleen significantly decreased in size and was palpable 5 cm below the left costal margin. Results of a complete blood cell panel were notable for a marked reduction of the white blood cell count to 6.3 K/UL with 70% segmented neutrophils, 0% bands, and 0% blasts. The patient was able to tolerate the tyrosine-kinase inhibitor therapy and will continue to be monitored for disease burden reduction.
Discussion. The estimated incidence of CML is 1 to 2 cases per 100,000 adults.1 The median age of onset is between 45 and 55 years, and the diagnosis is often made via incidental findings.2,3 CML represents 15% of all leukemia cases diagnosed yearly with a mortality rate of 1% to 2% since the introduction of imatinib in 2000.2,3 The 5-year survival rate of patients with CML has increased significantly from 22% in the 1970s to 70% from 2009 to 2015, since the introduction of imatinib.2-4 Since 2010, there has been an increased interest in second-generation tyrosine-kinase inhibitors, dasatinib and nilotinib, which have shown increased likelihood of achieving treatment-free remission.1,4 The length of treatment has been a subject of debate, but there are multiple ongoing studies to investigate further.5
CML is a myeloproliferative disorder of the hematopoietic stem cells that is caused by a balanced translocation of the long arms of chromosomes 9 and 22.6 The diagnosis of CML is established by confirmation of the Philadelphia chromosome either by FISH or polymerase chain reaction testing.3 The disease has 3 phases—chronic, accelerated, and blast—with 85% to 90% of CML cases diagnosed during the chronic phase.3,7 Patients are often asymptomatic, and CML is typically diagnosed via incidental abnormal blood counts.3 According to World Health Organization criteria, patients who have a blast cell count of less than 10% are in the chronic phase, between 10% and 19% are in the accelerated phase, and greater than 20% are in the blast phase.3,9 Using these percentage cutoffs, our patient received a diagnosis of chronic CML, as her blast cell percentage was approximately 1%. The adverse prognostic factors include advanced age, a white blood cell count of more than 60,000/μL at diagnosis, presence of splenomegaly, increased blast or basophil counts, thrombocytosis or thrombocytopenia, and cytogenetic clonal evolution.3,10
Leukostasis is a rare but serious and often fatal complication of CML, which is usually indicative of the adverse prognostic features in patients with CML and has been associated with symptoms of decreased tissue perfusion.11,12 Leukostasis, otherwise known as hyperleukocytosis, occurs when the white blood cell count is greater than 100,000/μL and becomes symptomatic when the white blood cell count exceeds 400,000/μL.12 Patients often present with cardiac, respiratory, renal, or neurological deficits caused by tissue hypoxia.12 However, the exact cause of hypoxia is unknown.12 Possible theories include increased blood viscosity because of increased cell volume, which results in impeded blood flow, and increased cytokine production, which leads to increased cell adhesion receptors resulting in blocked microvessels by the recruited leukocytes.12
Our patient's significantly elevated white blood cell count of 700,000/μL resulted in leukostasis with symptoms of acute respiratory failure and hypoxia. As it is often seen in pulmonary embolism cases, patients with leukostasis can also have normal chest radiograph findings, as was in our case.12 Unfortunately, patients who present with pulmonary symptoms have the worst short-term prognosis.11,12 Treatment options are limited for these patients. Current treatment focuses on aggressive supportive care and cytoreduction with leukapheresis, hydroxyurea, and/or conventional induction chemotherapy.12
The current first-line treatment consists of tyrosine-kinase inhibitors, which are potent and selective inhibitors of the BCR/ABL tyrosine kinase, the molecular abnormality that causes CML.11 Tyrosone-kinase inhibitors are the initial medication of choice for treating CML; they have demonstrated a significant decrease in the incidence of the blast phase of CML.13 Imatinib is considered to be a standard of care for the treatment of CML.14 Second-generation tyrosine-kinase inhibitors such as dasatinib and nilotinib are excellent alternatives if the patient is unable to tolerate imatinib or if the CML exhibits imatinib resistance.14 For these patients, it is recommended that they remain on imatinib indefinitely.4 Studies have shown that patients taking a second-generation tyrosine-kinase inhibitor have a higher likelihood of achieving treatment-free remission compared with imatinib.3 Should the progression to the blast phase occur, it is known to be highly refractory to treatment, with a response rate to standard chemotherapy of approximately 20% and a rate of complete remission of less than 10%.15 There has been discussion about stopping therapy after the patient has achieved remission, but multiple studies have found that more than 50% of patients have disease recurrence after stopping therapy.16 Given our patient’s young age, white blood cell count at the time of diagnosis, and the risk of treatment failure, dasatinib was chosen over imatinib.
Pregnancy was another factor we considered for the lifetime treatment plan for our young patient given her age. One study of 180 pregnant women found that only 50% of patients taking imatinib during pregnancy had uneventful pregnancies, with the other 50% documented to have fetal abnormalities or spontaneous abortions.17 Thus, it is currently recommended that tyrosine-kinase inhibitors be discontinued prior to pregnancy. However, there is a risk of disease recurrence during that period of discontinued treatment.17,18 Another retrospective study of 17 pregnant women found that patients who achieved a major molecular response to therapy for more than 3.5 years had a higher likelihood of no disease recurrence during the pregnancy despite discontinuing the medication.18 Furthermore, the study results showed that most patients who had disease recurrence during pregnancy were able to again achieve a major molecular response after restarting therapy.18
Patients who progress to the blast phase of CML are challenging to manage. Some of the factors associated with poor prognosis are treatment with a tyrosine-kinase inhibitor before transformation to the blast phase of CML, age 58 years or older, lactate dehydrogenase level of 1227 IU/L or greater, platelet count of more than 102 K/UL.13 A combination of tyrosine-kinase inhibitors and chemotherapy, followed by stem cell transplantation significantly improves survival. Patients receiving allogeneic stem cell transplants at the time of blast-phase diagnosis demonstrate superior survival vs those not undergoing stem cell transplant (43% vs 13%, respectively).13
Conclusion. The risk of leukostasis is higher in the setting of myeloid leukemia with white blood cell counts greater than 100,000/µL. Leukapheresis and targeted therapy with intensive supportive care should be initiated promptly, since these measures can increase the chance of survival. More agents and treatment options are also being researched for improved outcomes. Our patient’s age and the prognostic features of the disease dictated a possible necessity for lifelong therapy. Treatment carries a significant challenge should pregnancy occur, which will require more research into the best treatment approach. Allogeneic stem cell transplantation is a last-resort treatment option for patients who are unable to achieve disease remission.
References
1. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93(3):442-459. https://doi.org/10.1002/ajh.25011
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. https://doi.org/10.3322/caac.21590
3. Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164-172. https://doi.org/10.1056/nejm199907153410306
4. von Bubnoff N, Duyster J. Chronic myelogenous leukemia: treatment and monitoring. Dtsch Arztebl Int. 2010;107(7):114-121. https://doi.org/10.3238/arztebl.2010.0114
5. Talpaz M, Saglio G, Atallah E, Rousselot P. Dasatinib dose management for the treatment of chronic myeloid leukemia. Cancer. 2018;124(8):1660-1672. https://doi.org/10.1002/cncr.31232
6. Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303-2309. https://doi.org/10.1182/blood-2006-09-047266
7. Giles FJ, Cortes JE, Kantarjian HM, O'Brien SM. Accelerated and blastic phases of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18(3):753-xii. https://doi.org/10.1016/j.hoc.2004.03.005
8. Bonifacio M, Stagno F, Scaffidi L, Krampera M, Di Raimondo F. Management of chronic myeloid leukemia in advanced phase. Front Oncol. 2019;9:1132. https://doi.org/10.3389/fonc.2019.01132
9. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017.
10. Garcia-Manero G, Faderl S, O'Brien S, Cortes J, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer. 2003;98(3):437-457. https://doi.org/10.1002/cncr.11520
11. Leis JF, Primack SL, Schubach SE, Curtin PT, Druker BJ, Maziarz RT. Management of life-threatening pulmonary leukostasis with single agent imatinib mesylate during CML myeloid blast crisis. Haematologica. 2004;89(9):ECR30. https://doi.org/10.3324/%25x
12. Ali AM, Mirrakhimov AE, Abboud CN, Cashen AF. Leukostasis in adult acute hyperleukocytic leukemia: a clinician's digest. Hematol Oncol. 2016;34(2):69-78. https://doi.org/10.1002/hon.2292
13. Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. 2017;123(22):4391-4402. https://doi.org/10.1002/cncr.30864
14. Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398-2406. https://doi.org/10.1038/leu.2017.253
15. Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038-1042. https://doi.org/10.1056/nejm200104053441402
16. Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638-1647. https://doi.org/10.1038/leu.2016.115
17. Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505-5508. https://doi.org/10.1182/blood-2007-10-114900
18. Dou X, Qin Y, Huang X, Jiang Q. Planned pregnancy in female patients with chronic myeloid leukemia receiving tyrosine kinase inhibitor therapy. Oncologist. 2019;24(11):e1141-e1147. https://doi.org/10.1634/theoncologist.2019-0109
19. Craddock CF. We do still transplant CML, don't we? Hematology Am Soc Hematol Educ Program. 2018;2018(1):177-184. https://doi.org/10.1182/asheducation-2018.1.177
20. Radujkovic A, Dietrich S, Blok HJ, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: a retrospective study by the EBMT chronic malignancies working party. Biol Blood Marrow Transplant. 2019;25(10):2008-2016. https://doi.org/10.1016/j.bbmt.2019.06.028
21. Sacchi S, Kantarjian HM, O'Brien S, et al. Chronic myelogenous leukemia in nonlymphoid blastic phase: analysis of the results of first salvage therapy with three different treatment approaches for 162 patients. Cancer. 1999;86(12):2632-2641. https://doi.org/10.1002/(sici)1097-0142(19991215)86:12%3C2632::aid-cncr7%3E3.0.co;2-a


