Peer Reviewed
Chylothorax and Respiratory Failure Following Spontaneous Vaginal Delivery in a Patient with Pre-Eclampsia
A 37-year-old gravida two para one woman at 35 weeks and 3 days of gestation presented to labor and delivery triage with a two-day history of gestational hypertension, headaches, and visual disturbances.
History. Her significant medical history included major depressive disorder, alcohol use disorder, advanced maternal age, rubella non-immunity, anemia, and severe fetal growth restriction. Upon admission, clinical findings pointed to pre-eclampsia with severe features: blood pressure of 155/86 mm Hg, urine protein-to-creatinine ratio of 2.27, serum creatinine of 1.19 mg/dL, and thrombocytopenia with a platelet count of about 75,000 per mcL. Consequently, she was initiated on intravenous magnesium sulfate (6 g bolus then 20 g/500 mL at 25 mL/hr for 2 days), labetalol (20 mg intravenously then 200 mg orally twice daily for 7 days), and oral nifedipine (60 mg twice daily for 10 days).
Labor was induced, resulting in a vaginal delivery at 35 weeks and 5 days. Postpartum, she exhibited persistent hypertension. On postpartum day 2, she developed shortness of breath and low oxygen saturations which required oxygen support. By postpartum day 8 she developed signs of sepsis and acute hypoxic respiratory failure. Physical examination findings on postpartum day 8 included diminished breath sounds and dullness to percussion over the right lower lung field.
Diagnostic testing. A computed tomography (CT) scan of the chest demonstrated a large right-sided pleural effusion and bilateral airspace opacities (Figures 1 and 2). A thoracentesis was performed. The pleural fluid was milky in consistency and laboratory testing of the fluid showed a high triglyceride content of 474 mg/dL. Radiological findings showed multifocal ground glass opacities and septal thickening (pattern of “crazy paving”) with additional confluent consolidations (Figure 3). Subsequent bronchoalveolar lavage revealed hemosiderin-laden macrophages and atypical lymphocytes, but cultures remained negative, although a respiratory polymerase chain reaction was reactive for Haemophilus influenzae. She also had an elevated b-type naturetic peptide level of 484 pg/mL.

Figure 1a. The posteroanterior view of the chest X-ray demonstrating a large right-sided pleural effusion (orange overlay) with bilateral patchy airspace opacities (solid arrows).

Figure 1b. The lateral view of the chest X-ray demonstrating a large right-sided pleural effusion (orange overlay).
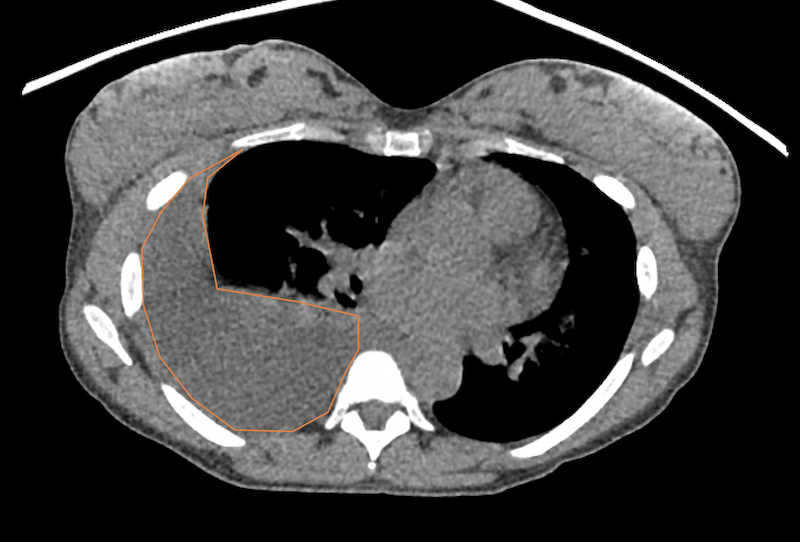
Figure 2a. Non-contrast chest CT scan with soft tissue windows confirming the presence of a large right pleural effusion (orange overlay).
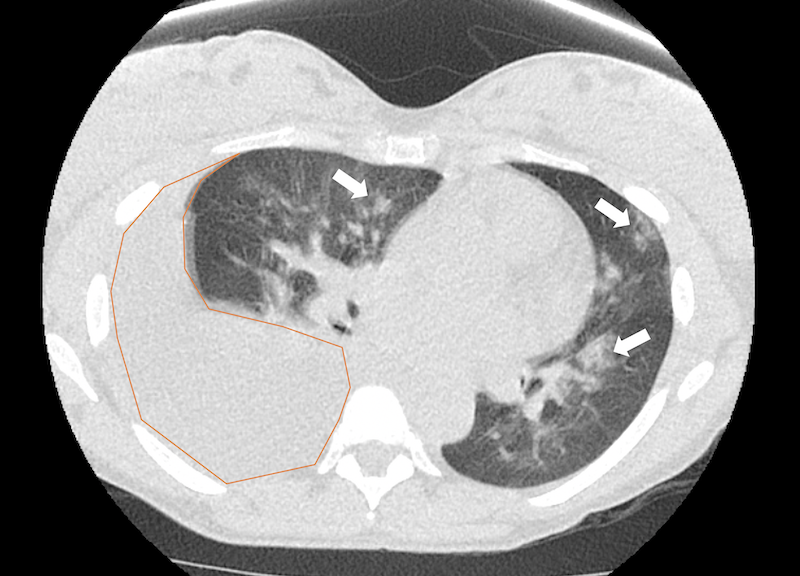
Figure 2b. Non-contrast chest CT scan with lung windows confirming the presence of a large right pleural effusion (orange overlay) with bilateral patchy airspace opacities (solid arrows).
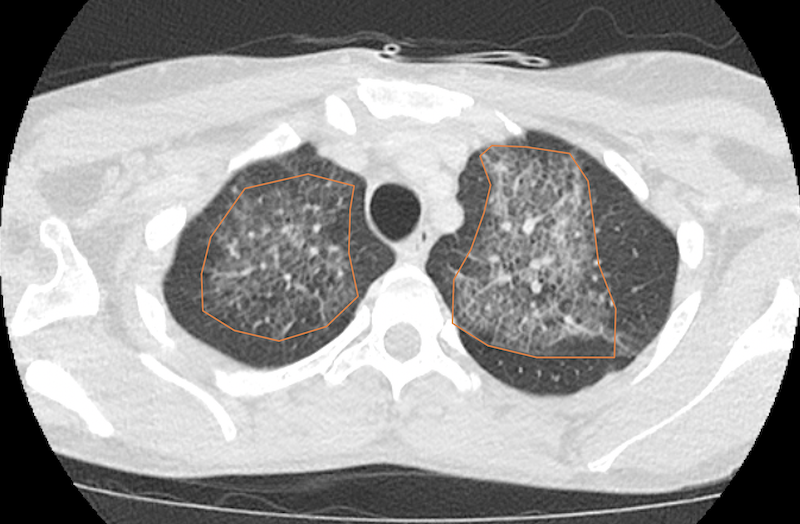
Figure 3a. Chest CT scan with intravenous contrast showing multifocal ground glass opacities and septal thickening (crazy paving appearance, orange overlay).

Figure 3b. Inferior view of the chest CT scan with intravenous contrast showing multifocal ground glass opacities and septal thickening (crazy paving appearance, orange overlay).
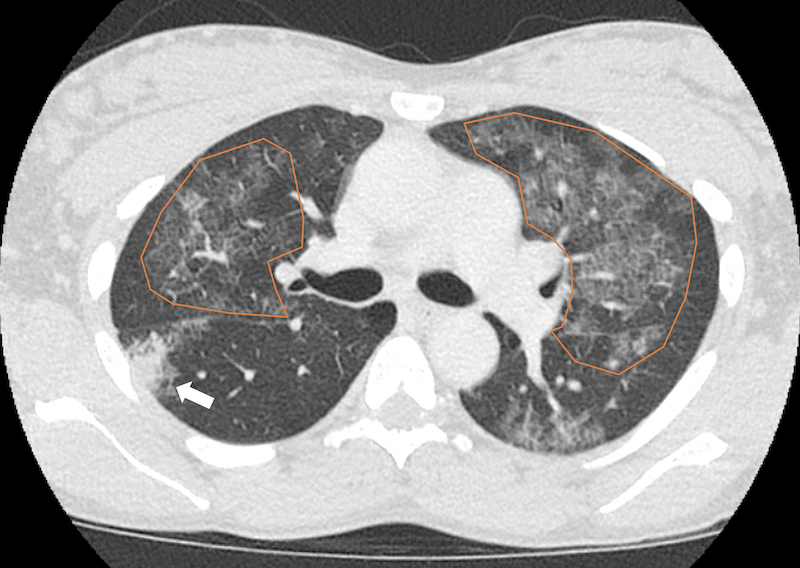
Figure 3c. Chest CT scan with intravenous contrast showing multifocal ground glass opacities and septal thickening (crazy paving appearance, orange overlay) with more confluent consolidations in the right middle lobe (solid arrow).

Figure 3d. Chest CT scan with intravenous contrast showing multifocal ground glass opacities and septal thickening (crazy paving appearance, orange overlay) with more confluent consolidations in the bilateral lower lobes (solid arrows).
Differential diagnoses. In evaluating the patient's respiratory symptoms and the large right-sided pleural effusion, several potential diagnoses were considered. The milky appearance of the pleural fluid with the high triglyceride content suggested chylothorax, while the radiological findings of airspace opacities prompted considerations of pulmonary edema, atypical pneumonia, and pulmonary alveolar proteinosis. Other differential diagnoses included cardiogenic pulmonary edema due to postpartum cardiomyopathy and infectious processes. While these other diagnoses were feasible in this clinical context and could produce the radiographic findings seen, chylous pleural fluid would not be expected in any of them. The pleural fluid analysis confirmed the diagnosis of chylothorax. Some of these conditions could have been occurring simulataneously, but were not the major cause of her respiratory symptoms.
Treatment and management. Throughout her admission, she required significant respiratory support, including oxygen and eventually intubation. A pleural drainage catheter was inserted, draining 1.3 L of chyle. She was admitted to the medical intensive care unit and managed with subcutaneous octreotide 50 mcq every 8 hours for 7 days and a fat-restricted diet. She improved over the course of 1 week with decreasing chest tube output.
Outcome and followup. Her condition improved, with decreased triglyceride content in the pleural fluid and improved pulmonary edema on imaging at 1 week. Concurrently, she developed acute kidney injury secondary to presumed pre-eclampsia, for which she was scheduled to follow-up with a nephrology specialist on an outpatient basis.
Discussion. Chylothorax is characterized by chyle accumulation in the pleural space. Chyle is a lymphatic fluid rich in chylomicrons, immunoglobulins, lymphocytes, and enzymes and is primarily transported by the thoracic duct. The thoracic duct delivers up to four liters of chyle daily from the intestines to the left subclavian vein.1,2 Typically, chyle leaks are categorized into causes such as traumatic, congenital, or postoperative. Chylothorax often results from trauma, particularly iatrogenic events such as surgeries or central venous catheterization. Non-iatrogenic causes from trauma include penetrating injuries.2 Although the primary etiology of chylothorax revolves around trauma or surgery, cases have emerged without clear traumatic incidents.3 Non-traumatic non-iatrogenic causes include infections, neoplasms, inflammatory processes, and increased intraductal pressures.2 Spontaneous occurrences have been reported post-coughing or sneezing.4 A rare instance of chylothorax arises during childbirth, likely due to increased intrathoracic pressures from the Valsalva maneuver during labor causing duct rupture.5,6 In cases without malignant or infectious duct disruptions, inherent duct malformations or weaknesses could predispose individuals to spontaneous rupture.6 Some patients have shown thoracic duct anomalies predisposing them to lymphatic vessel rupture under increased pressures or spinal hyperextension.7
Chylothorax associated with pregnancy is an exceedingly rare event, with very few reported instances in literature.6,8-10 The Valsalva maneuver, employed during childbirth, results in high intra-abdominal and intrathoracic pressures. It is postulated that this can precipitate chylothorax due to lymphatic vessel rupture, particularly in the presence of small transdiaphragmatic defects.11,12 The pathophysiology of chylothorax during delivery could potentially be analogous to the mechanism seen in patients with decompensated cirrhosis, where ascites causes functional obstruction of the thoracic duct due to raised intra-abdominal pressure.12
Despite their high lipid content, chylothoraces are highly variable in their CT attenuation due to their high protein content, occasional hemorrhagic components, and lack of detectable fat in CT. Therefore, a thoracentesis should be performed if a diagnosis of chylothorax or pseudochylothorax is suspected.13 The observed "crazy paving" pattern on CT, initially linked with pulmonary alveolar proteinosis, generally represents partial filling of the alveoli. Ground glass opacities and septal thickening are common features.14 In this case, the crazy paving pattern was likely caused by pulmonary edema secondary to sequelae of pre-eclampsia such as capillary permeability and cardiac dysfunction, supported by her elevated B-type natriuretic peptide (BNP) test. With her granulocyte-rich bronchoalveolar lavage, positive H. influenzae test, and other imaging findings, there was likely concomitant atypical pneumonia.15-17
Prior cases of postpartum chylothorax have varied in their presentations. For instance, the 1987 case from Tornling and colleagues involved external abdominal pressure during delivery.8 Other cases have pointed to high intrathoracic pressures or undetected pre-existing effusions.6,9 A 2022 case stands out, as the patient presented postpartum revealing an anatomically unique thoracic duct.10 Another case reported bilateral chylothorax several months post-delivery, remedied through video-thoracoscopy and talc pleurodesis.18
The diagnosis of chylothorax can be made with analysis of pleural fluid. Magnetic resonance lymphangiography or lymphoscintigraphy may be considered for detecting contributory lymphatic abnormalities.19 The management of postpartum chylothorax after pregnancy is nebulous due to its rarity. In our case, management with pleural drainage, octreotide, and a low-fat diet resulted in a positive clinical outcome. Conservative treatments can be initiated, but if they fail, surgical or other measures such as lymphoscintigraphy with subsequent embolization might be warranted.20
Postpartum chylothorax, although rare, presents a diagnostic and therapeutic challenge in obstetric medicine. As demonstrated in this case, a comprehensive clinical evaluation combined with targeted therapeutic interventions can lead to favorable outcomes. Continued documentation of such cases will further elucidate the optimal management strategy for this uncommon complication.
References:
1. Rudrappa M, Paul M. Chylothorax. StatPearls Publishing; 2023. Accessed September 7, 2023. https://pubmed.ncbi.nlm.nih.gov/29083798/
2. Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg. 1997;84(1):15-20. doi:10.1046/j.1364-2168.1997.02654.x
3. Doerr CH, Allen MS, Nichols FC, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005;80(7):867-870. doi:10.4065/80.7.867.
4. Fornés S, Bielsa S, Porcel JM. Sneeze-induced chylothorax. Arch Bronconeumol. 2021;57(8):558-559. doi:10.1016/j.arbr.2021.05.017.
5. Li X, Gu YU, Zhu LEI, Cheng W, Pan M, Zhuang Y. Pregnancy complicated by spontaneous chylothorax: a case report. Acta Obstet Gynecol Scand. 2006;85(12):1510-1511. Doi:10.1080/0001634060060912.
6. Cammarata SK, Brush RE, Hyzy RC. Chylothorax after childbirth. Chest. 1991;99(6):1539-1540. doi:10.1378/chest.99.6.1539.
7. Schwartz J. Spontaneous chylothorax. JAMA. 1960;174(16):2033-2035. Doi:10.1001/jamma.1960.03030160019005.
8. Tornling G, Axelsson G, Peterffy A. Chylothorax as a complication after delivery. Acta Obstet Gynecol Scand. 1987;66(4):381-382. Doi:10.3109/00016348709103660
9. Konstantinidis K, Soultanis K, Gouma A, Siafakas K. Spontaneous chylothorax identified during delivery. J Cardiothorac Surg. 2015;10:A266. doi:10.1186/1749-8090-10-S1-A266.
10. Streit A, Seitlinger J, Siat J, Renaud S. Chylothorax following childbirth: a rare complication where interventional treatment seems mandatory. Clin Case Rep Int. 2022;6(1):1296. Doi:10.25107/2638-4558.1296.
11. Sharpey-Schafer EP. Effects of Valsalva's manoeuvre on the normal and failing circulation. Br Med J. 1955;1(4915):693-695. doi:10.1136/bmj.1.4915.693.
12. McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104(1):1-8. doi:110.1016/j.rmed.2009.08.010.
13. Hooper C, Lee YCG, Maskell N, et al; BTS Pleural Guideline Group
Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65:4-17. Doi:10.1136/thx.2010.136978.
14. De Wever W, Meersschaert J, Coolen J, Verbeken E, Verschakelen JA. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging. 2011;2(2):117-132. doi:10.1007/s13244-010-0060-5.
15. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785-799. Doi:10.1016/S0140-6736(05)17987-2.
16. Vázquez-Rodríguez JG, Veloz-Martínez MG. Pleural effusion and ascites in severe preeclampsia: frequency and correlation with plasma colloid osmotic pressure and renal filtration function. Cir Cir. 2011;79(4):299-305.
17. Castaneda C, Magh A, Powel J. Pre-eclampsia-associated pleural effusion in the postpartum period: a peculiar precipitant of hypoxia in parturients. Chest. 2018;154(4):385A. doi:10.1016/j.chest.2018.08.352.
18. Honguero Martínez A, Obrer AA, Alonso DP, Guerrero ME, Alcaide CM, Cantó Armengod A. Bilateral chylothorax after delivery: an infrequent case treated with videothoracoscopic talc pleurodesis. Cir Esp. 2006;80:400–402. Doi:10.1016/S0009-739X(06)70994-0.
19. Notohamiprodjo M, Weiss M, Baumeister RG, et al. MR lymphangiography at 3.0 T: correlation with lymphoscintigraphy. Radiology. 2012;264(1):78-87. doi:10.1148/radiol.12110229.
20. Schild HH, Strassburg CP, Welz A, Kalff J. Treatment options in patients with chylothorax. Dtsch Arztebl Int. 2013;110(48):819-826. doi:10.3238/arxtebl.2013.0819.
AFFILIATIONS:
1University of Florida College of Medicine, Gainesville, FL
2University of South Carolina School of Medicine Columbia, Columbia, SC
3Department of Radiology, University of Florida College of Medicine, Gainesville, FL
CITATION:
McCann UG, Mirza A, Mathelier M, et al. Chylothorax and respiratory failure following spontaneous vaginal delivery in a patient with pre-eclampsia. Consultant. 2024;64(2):e2. doi:10.25270/con.2024.01.000004
Received October 10, 2023. Accepted December 5, 2023. Published online January 23, 2024.
DISCLOSURES:
The authors report no relevant financial relationships.
ACKNOWLEDGEMENTS:
None.
CORRESPONDENCE:
George McCann, University of Florida College of Medicine, 1600 SW Archer Road, Gainesville, FL 32610 (umccann@ufl.edu)


