Peer Reviewed
Shear-Wave Elastography in the Evaluation of Thyroid Nodules and Correlation With Aggressive Features on Microscopy
AUTHORS:
Debbie W. Chen, MD1 • Julie Horst, MD2 • Devraj Basu, MD, PhD3 • Sharvari Dalal, MD4 • Darshana Jhala, MD, BMus5 • Kristen A. Hyland, MD, MHS, ENCU6
AFFILIATIONS:
1Department of Internal Medicine, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania
2Department of Pathology, Wilmington VA Medical Center, Wilmington, Delaware
3Department of Otolaryngology–Head and Neck Surgery, Hospital of the University of Pennsylvania; and Department of Surgery, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
4Department of Pathology and Laboratory Medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
5Department of Clinical Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine; and Department of Pathology and Laboratory Medicine, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
6Department of Medicine, Wilmington VA Medical Center, Wilmington, Delaware
Thyroid nodules are common in the United States, with a prevalence of 50% or more in the adult population as detected by way of neck palpation or ultrasonography (US) or at autopsy.1,2 Most thyroid nodules are benign (80% are colloid nodules, cysts, or thyroiditis; 10%-15% are benign follicular neoplasms), with approximately 5% found to be malignant.1 Specific sonographic features such as microcalcifications, irregular or spiculated margins, hypoechogenicity, and intramodular blood flow increase the suspicion that the nodule is cancerous. Nodules greater than 1 cm in diameter on US should be biopsied for further cytologic evaluation.3
Shear-wave elastography (SWE) is a relatively new and dynamic technique that has been developed to quantitatively evaluate the elasticity of tissues. SWE uses an acoustic US probe to stimulate the tissue and then calculate the propagation speed of the resultant transverse-oriented shear waves through the tissue.4-6 The stiffer the tissue, the faster the shear wave will travel through. Assessment of the tissue elasticity is derived from the shear-wave velocity (SWV), expressed in meters per second.7 SWV is greater in hard (less elastic) tissue, and lesser in soft (more elastic) tissue.5 SWE also creates qualitative color-coded elastograms that display soft tissue in blue and stiffer tissue in red.8
Case Report
A 71-year-old man presented for evaluation of a thyroid nodule. The nodule had been found incidentally on carotid US and magnetic resonance imaging of the brain, which he had undergone as part of a workup for balance problems.
History. The man was a military veteran with a medical history significant for hypertension, hyperlipidemia, and type 2 diabetes mellitus. He had no history of head or neck radiation exposure, no known family history of thyroid cancers, and no compressive symptoms.
Physical examination. Neck palpation revealed a small, hard nodule in the thyroid isthmus that moved with swallowing, but no lymphadenopathy.
Diagnostic tests. His thyrotropin level was normal (0.495 mIU/L; reference range, 0.49-4.67 mIU/L). Findings of initial neck US performed at an outside hospital identified a solitary right thyroid nodule. Repeated US revealed 3 nodules that were greater than 1 cm in diameter and a few subcentimeter-sized nodules. In the central aspect of the right lobe was an ill-defined, 3.1-cm, heterogeneous, isoechoic solid nodule with mildly irregular margins, microcalcifications, a taller-than-wide shape, and increased vascularity (Figure 1). In the left aspect of the isthmus was a well-circumscribed, 1.3-cm, ovoid solid nodule with internal microcalcifications, a wider-than-tall shape, and peripheral vascularity (Figure 2).
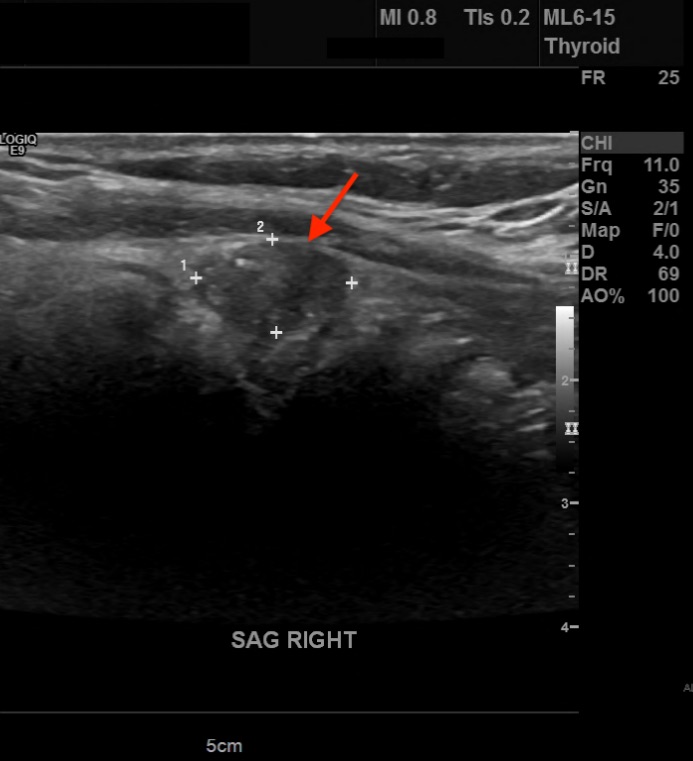
Figure 1. A 3.1-cm, ill-defined, heterogeneous, isoechoic solid nodule (arrow) with irregular margins, microcalcifications, and a taller-than-wide shape in the right lobe of the thyroid.
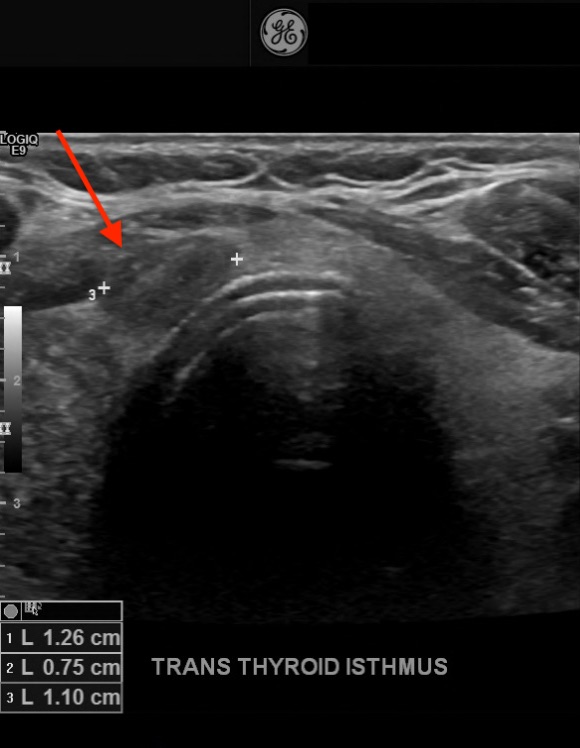
Figure 2. A 1.3-cm, well-circumscribed, solid nodule (arrow) with microcalcifications and a wider-than-tall shape in the isthmus of the thyroid.
In the lateral aspect of the left lobe was a 1.1-cm, isoechoic solid nodule with rim shadowing calcifications, a taller-than-wide shape, and mildly increased internal vascularity. Routine SWE was performed using Virtual Touch imaging on a Siemens ACUSON S2000 ultrasound system. The right nodule had 2 areas of high SWV measuring 3.88 m/s and 2.07 m/s; the surrounding thyroidal tissue had a SWV of 2.06 m/s. The isthmus nodule exhibited higher SWV in the medial aspect, with an area measuring 4.56 m/s; the lateral aspect of the nodule had a SWV of 2.71 m/s; and the surrounding thyroidal tissue had a SWV of 2.46 m/s (Figure 3).
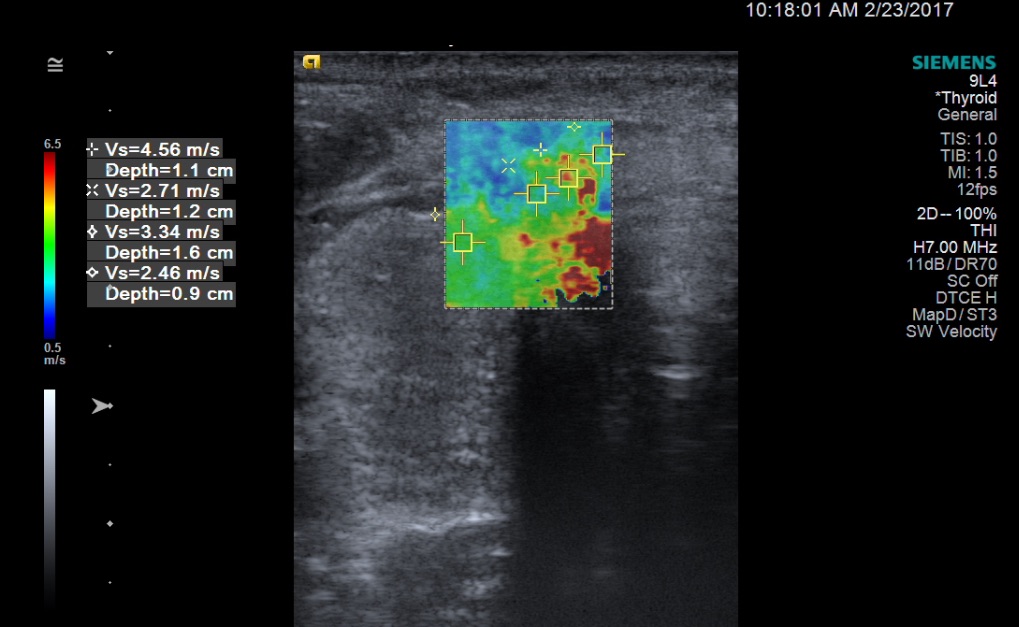
Figure 3. SWE image (soft tissue in blue, stiffer tissue in red) with quantitative SWV measurements of the isthmus nodule. In the medial aspect of the isthmus nodule is an area that exhibited a high SWV of 4.56 m/s. The lateral aspect of the nodule had a SWV of 2.71 m/s, and the surrounding thyroidal tissue had a SWV of 2.46 m/s.
Fine-needle aspiration of the right dominant and isthmus nodules was performed. Cytologically, the nodules were consistent with a benign follicular nodule and a papillary thyroid carcinoma, respectively (Figures 4-6).
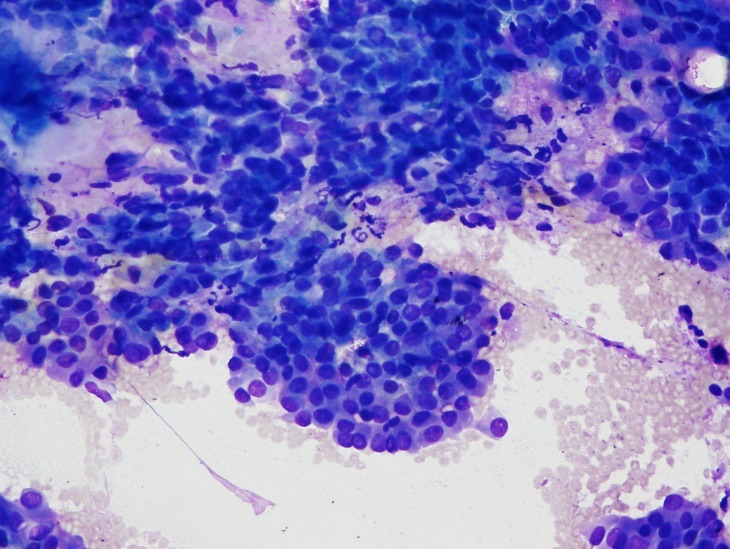
Figure 4. Fine-needle aspiration cytology from the less-elastic area of the thyroid isthmus nodule showing a cellular aspirate (Diff-Quik stain, low-power view).
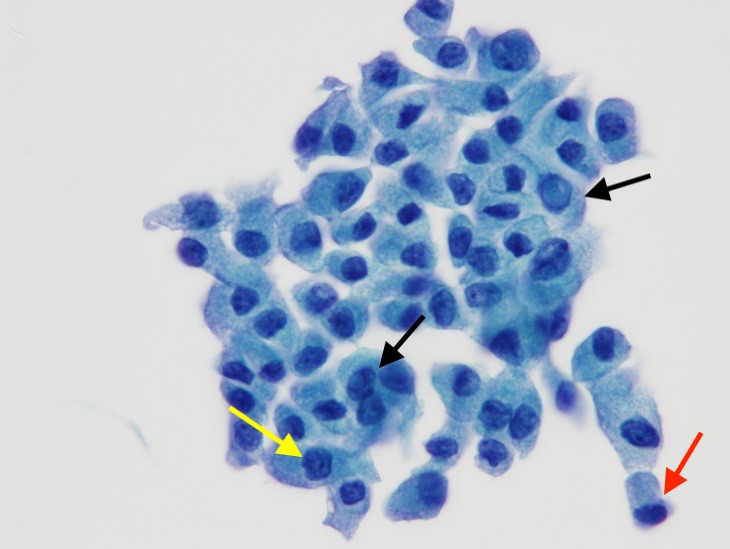
Figure 5. Papanicolaou stain, intermediate-power view, showing cells with intranuclear inclusion (black arrows), and one cell with a nuclear groove (yellow arrow). A taller-than-wide epithelial cell (red arrow) is also present.
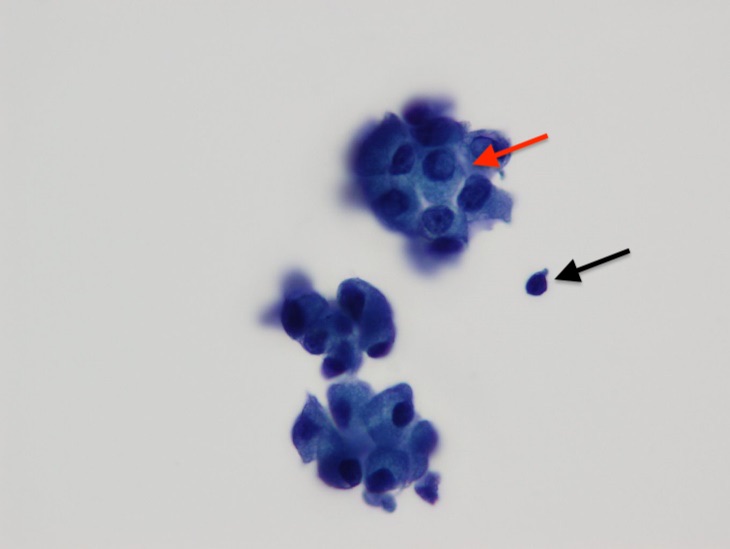
Figure 6. Papanicolaou stain, high-power view, showing cells in 3-dimenstional groupings with a cell containing intranuclear inclusions (red arrow). A lymphocyte (black arrow) is present for size comparison.
The patient underwent a total thyroidectomy without lymph node dissection. Pathologically, the right thyroid lobe was benign, with no evidence of active malignancy. A 7-mm nodule in the isthmus showed papillary thyroid microcarcinoma with 25% tall-cell morphology and evidence of focal extrathyroidal extension without angioinvasion or lymphatic invasion (Figures 7 and 8).
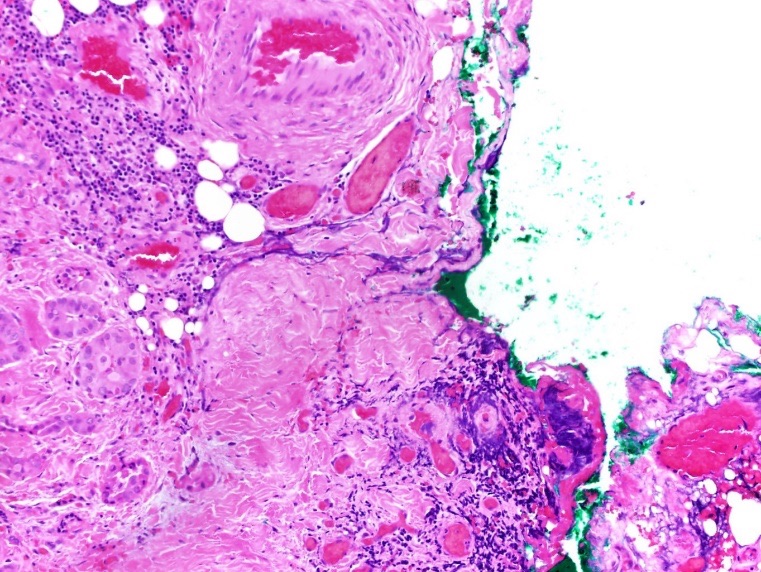
Figure 7. Hematoxylin-eosin stained pathology of papillary thyroid carcinoma in the isthmus nodule, intermediate-power view (×200) showing tall tumor cells with abundant eosinophilic cytoplasm (aggressive variant of papillary thyroid carcinoma) with focal extrathyroidal extension.
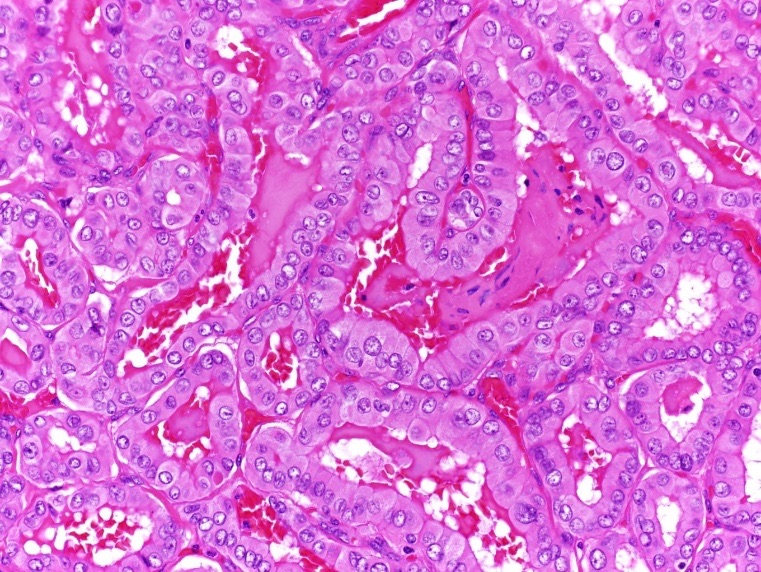
Figure 8. Hematoxylin-eosin stain, high-power view (×400) showing the nuclear features of the papillary thyroid cancer.
Postoperatively, the patient underwent recombinant human thyrotropin–stimulated radioactive iodine (RAI) ablation. Before RAI ablation, radioiodine whole-body scintigraphy demonstrated a focus of intense tracer activity in the right neck and a smaller uptake in the left neck (Figure 9). The post-RAI ablation scan demonstrated tracer uptake in the neck consistent with iodine-avid tissue of thyroid origin with no evidence of distant metastasis (Figure 10).
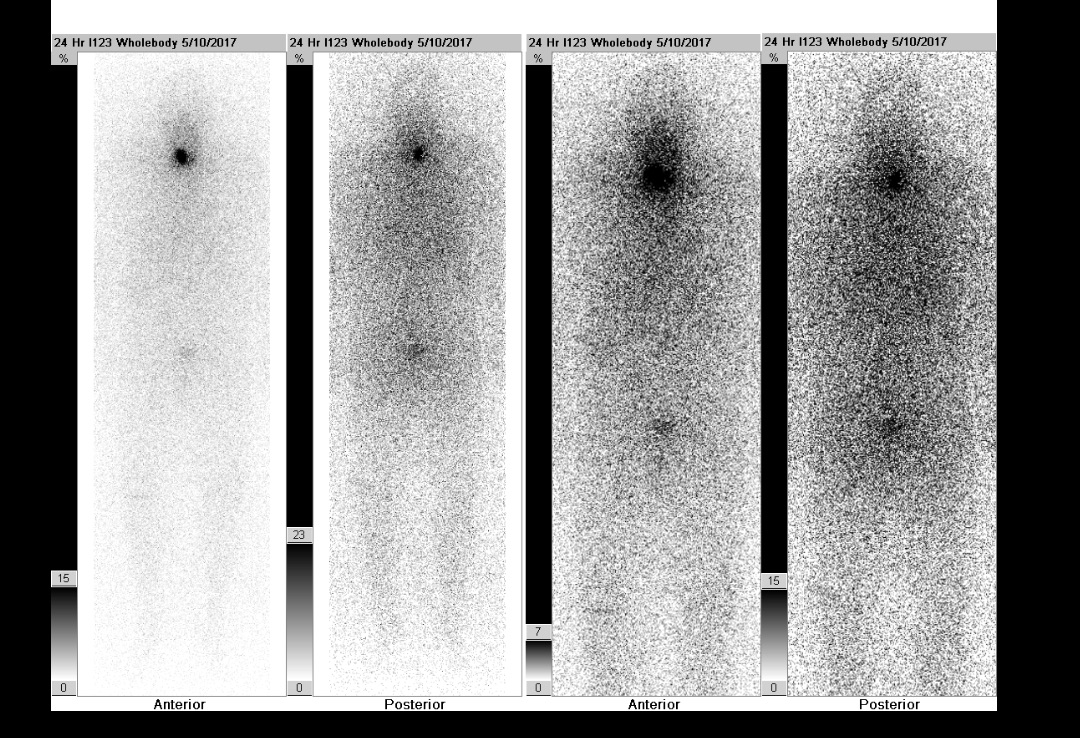
Figure 9. Pre-RAI ablation uptake scan demonstrated a focus of intense tracer activity in the right neck and a smaller uptake in the left neck.
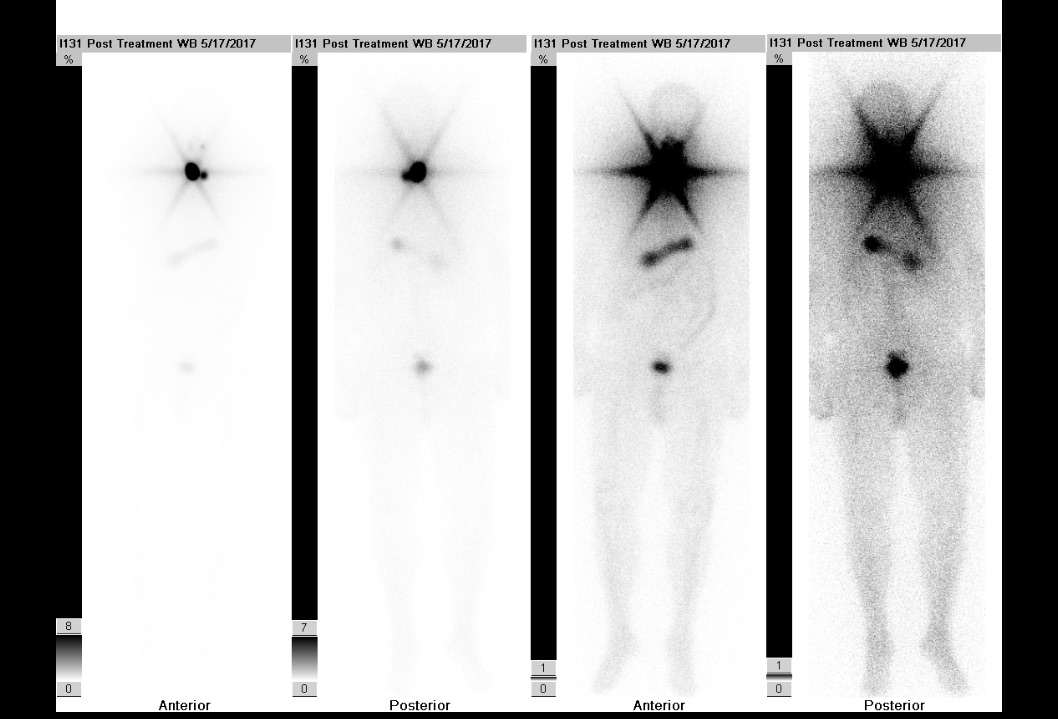
Figure 10. Post-RAI ablation uptake scan demonstrated tracer uptake in the neck consistent with iodine-avid tissue of thyroid origin with no evidence of distant metastasis.
Outcome of the case. The patient had an excellent response to therapy, with negative thyroglobulin antibodies and a thyroglobulin level of less than 0.5 ng/mL at follow-up, decreased from 92.5 ng/mL (reference range, 1.4-29.3 ng/mL) 3 weeks after RAI ablation. He was placed on maintenance therapy with levothyroxine, 175 µg once daily. This excellent response was maintained at follow-up 1 year after RAI ablation, with undetectable thyroglobulin antibodies, a stable thyroglobulin level, and a suppressed thyrotropin level of 0.10 mIU/L.
Discussion
This case demonstrates the clinical utility of SWE, which is operator-independent and reproducible, as an adjunctive tool to conventional US in identifying thyroid nodules with high concern for malignancy, and to facilitate the localization of high-yield areas of thyroid nodules to biopsy. Sonographic features of the right thyroid nodule were more highly suspicious for malignancy compared with those of the isthmus nodule. Evaluation with SWE, however, showed that the isthmus nodule was relatively more concerning for malignancy based on its higher SWV (4.56 m/s compared with 3.88m/s in the right nodule). The pathology results showed that the nodule in the right lobe was benign and the nodule in the isthmus was malignant, findings that are more consistent with the level of suspicion based on the nodules’ elastic properties than their sonographic features.
This is the first study correlating higher SWV and corresponding aggressiveness of the tumor, which could have implications on management and future therapeutic studies.
Primarily in the radiology literature, studies have investigated the role of SWE to help distinguish malignant from benign thyroid nodules. Rago and colleagues9 were the first to report on the clinical application of strain elastography, which evaluates elasticity through tissue displacement caused by compression, on thyroid nodules. They showed that nodules with decreased elasticity were associated with an increased risk of malignancy.
Sebag and colleagues8 reported on the utility of SWE to predict malignancy in thyroid nodules in an analysis of 146 thyroid nodules from 93 patients. They demonstrated that the elasticity index of malignant nodules was significantly higher than that of benign nodules and normal thyroid glandular tissue. In another study, the sensitivity of predicting malignancy in a thyroid nodule was 1.5 times higher when a combination of elasticity characteristics and classic sonographic features was used compared with the use of only the sonographic features.10 Using multivariate analysis, Azizi and colleagues11,12 showed that thyroid nodular stiffness, measured by strain and by SWE, is an independent predictor of thyroid cancer.
Because malignant lesions are often associated with mechanical changes in the affected tissue and are more likely to be less-elastic than benign lesions, SWE has great promise as a complementary tool to conventional US in the evaluation of thyroid nodules. SWE can be especially useful in the evaluation of indeterminate nodules by identifying areas of decreased elasticity to biopsy.
References:
- Hegedüs L. The thyroid nodule. N Engl J Med. 2004;351(17):1764-1771.
- Veloski C, Siraj ES. A young woman with a thyroid nodule. J Clin Outcomes Manag. 2008;15(9):443-449.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
- Ophir J, Alam SK, Garra B, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213(3):203-233.
- Kwak JY, Kim E-K. Ultrasound elastography for thyroid nodules: recent advances. Ultrasonography. 2014;33(2):75-82.
- Gennisson J-L, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94(5):487-495.
- Jeong WK, Lim HK, Lee H-K, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33(3):149-160.
- Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95(12):5281-5288.
- Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92(8):2917-2922.
- Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20(4):260-274.
- Azizi G, Keller J, Lewis M, Puett D, Rivenbark K, Malchoff C. Performance of elastography for the evaluation of thyroid nodules: a prospective study. Thyroid. 2013;23(6):734-740.
- Azizi G, Keller JM, Mayo ML, et al. Thyroid nodules and shear wave elastography: a new tool in thyroid cancer detection. Ultrasound Med Biol. 2015;41(11):2855-2865.


