Peer Reviewed
Irritable Bowel Syndrome: Practical Considerations for Nurse Practitioners and Physician Assistants
Authors:
Sarah Patel, MS, PA-C, MBA
Rutgers University, Piscataway, New Jersey; Weill Cornell Medical Center, New York, New York
Elayne DeSimone, PhD, NP-C
Widener University School of Nursing, Chester, Pennsylvania
Bethany M. Doerfler, MS, RDN
Northwestern Medicine, Chicago, Illinois
Albena Halpert, MD
Boston University School of Medicine, Boston, Massachusetts
Susan Lucak, MD
Weill Cornell Medical Center, New York, New York
Citation:
Patel S, DeSimone E, Doerfler BM, Halpert A, Lucak S. Irritable bowel syndrome: practical considerations for nurse practitioners and physician assistants [published online November 27, 2018]. Consultant360.
Disclosures:
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors take full responsibility for the scope, direction, and content of the manuscript and have approved the submitted manuscript. The authors received no compensation related to the development of the manuscript. They would like to thank Helen Woodroof, PhD, of Complete HealthVizion, Inc, for editorial assistance in the writing and revision of the draft manuscript on the basis of detailed discussion and feedback from all the authors; this assistance was funded by Allergan plc.
Financial Disclosures:
Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Sarah Patel, Elayne DeSimone, Albena Halpert, and Susan Lucak have received honoraria for their roles as consultants to Allergan plc. Bethany M. Doerfler has received honoraria for her role as a consultant to Allergan plc and has received honoraria from Allakos and Nutricia North America.
The concept for this article was originally discussed at a meeting convened by Allergan plc that discussed topics for physician education on irritable bowel syndrome, for which participants, including the authors on this publication, received an honorarium. The authors received no payment in relation to the development of this publication, which was developed separately from the meeting.
ABSTRACT: Irritable bowel syndrome (IBS) is a common, chronic gastrointestinal tract disorder that accounts for approximately 10% to 15% of primary care visits. While IBS can be highly debilitating, patients may be reluctant to discuss their symptoms with a health care provider. Due to their prominent position in day-to-day patient care, nurse practitioners and physician assistants can play a key role in the prompt diagnosis and provision of effective care for patients with IBS and are well placed to develop open and trusting relationships. This review combines the latest evidence with practical advice related to the diagnosis and management of IBS.
KEYWORDS: Irritable bowel syndrome, nurse practitioners, physician assistants
Why IBS Matters
Irritable bowel syndrome (IBS) is one of the most prevalent gastrointestinal (GI) tract disorders seen in primary care and results in significant morbidity and high health care costs.1,2 Nurse practitioners (NPs) and physician assistants (PAs) are often on the front line of managing this chronic condition and play a pivotal role in the diagnosis and ongoing care of patients. As such, it is important that NPs and PAs practicing in settings including primary care, emergency medicine/urgent care centers, gastroenterology, and retail health are familiar with the latest evidence related to the diagnosis and management of IBS.3 In this review, we present current approaches to the efficient diagnosis and multifactorial management of IBS.
IBS is the most commonly diagnosed GI tract disorder, affecting approximately 11% of the global population and 10% to 15% of the US population.4,5 Furthermore, IBS is the 7th most common diagnosis made by primary care physicians and accounts for approximately 10% to 15% of primary care visits.1,2,6 Both men and women are affected, but the overall incidence is 1.5- to 3-fold higher in women, although this may reflect differences in health care-seeking behavior.4,5,7 Most patients will experience symptoms before age 35 years; however, IBS can occur in patients of any age.4,7 IBS can be further classified according to the predominant bowel habit, with subtypes including IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with a mixed stool pattern (IBS-M), and unclassified IBS (IBS-U), where the patient’s bowel habits cannot be accurately categorized.8
IBS has a significant impact on patients’ quality of life, with a level of impairment similar to other chronic disorders such as depression and gastroesophageal reflux disease.9,10 In addition, patients report that IBS substantially interferes with personal activities and work performance and productivity. On average, patients report missing 2 days of school or work and 9 days of impacted productivity per month due to IBS.6 The average total health care cost for patients with IBS-D is $13,038 per patient per year, attributable to emergency department visits, hospitalizations, outpatient services, and prescription medications.11
A prompt diagnosis is important; patients with IBS whose symptoms are not initially correctly diagnosed may experience unnecessary diagnostic testing and even hospitalization and surgery, with rates of abdominal surgery, including cholecystectomy, appendectomy, and hysterectomy, being higher in patients with IBS than in the general population.10,12,13 After a diagnosis is established, appropriate multifactorial management strategies should be implemented. Many patients with IBS are dissatisfied with traditional treatment options that address only a single symptom, which can potentially result in numerous physician consultations, multiple failed treatment options, use of unproven therapies, and substantial costs.10,13 In fact, patients with IBS-D report using an average of 4.9 medications, with only 20% reporting satisfaction with previous treatments.14
What Causes IBS?
The pathophysiology of IBS is influenced by multiple factors, and identical symptoms may be caused by different underlying processes.1,15,16 Both environmental and patient factors are known to contribute to the symptoms of IBS (Figure 1). Environmental factors may include early life stressors, diet, and prior GI tract infections. Patient factors may include genetics, small-intestinal bacterial overgrowth, disturbances in the colon microbiota, mucosal inflammation and immune activation, changes in intestinal permeability, alterations in bile salt metabolism, abnormalities in serotonin metabolism, and alterations in brain functioning.1,15,16 A combination of pathophysiological mechanisms that are involved to differing degrees in different patients are thought to result in the typical constellation of symptoms seen in IBS, such as altered bowel habits, abdominal pain, bloating, and nausea. Food allergies are not known to be a cause of IBS, but symptoms may be triggered or exacerbated by certain foods in some cases.17-19

Abbreviations: GI, gastrointestinal; IBS, irritable bowel syndrome.
IBS is associated with a high degree of comorbidity, including systemic disorders (chronic fatigue, migraines, functional dyspepsia, fibromyalgia, interstitial cystitis, and restless legs syndrome) and psychosocial disorders (anxiety, stress, depression, and somatization), highlighting the need for a multidisciplinary approach, particularly in cases of significant comorbidity.16,20-24 Other psychosocial factors such as acute stress and history of sexual or physical abuse are also associated with an increased risk of IBS.1,16,21 However, it is important to note that IBS is a standalone diagnosis and is not simply secondary to anxiety or stress. In many patients with both IBS and mood disorders, there is evidence to suggest that in a major subset of patients, GI tract symptoms occur before the onset of mood disorders.15
Making a Diagnosis of IBS
Communication Is Key
Many patients with IBS suffer for years before discussing their symptoms with a health care provider (HCP)6; they may feel embarrassed and misunderstood by their family and HCPs, so establishing effective communication is important.1,6,25,26 People experiencing IBS symptoms often perceive stigma from HCPs and therefore may not volunteer information on their symptoms, even when they are having a negative impact on their quality of life.25,27 Patients with IBS, particularly those with severe symptoms, often feel that communication with their HCP is lacking and feel frustrated with their condition and lack of response to treatments. As a result, many patients resort to the internet or friends and family for information.6,28-30
The vigilant NP or PA will consider the barriers to a conversation regarding bowel habits and ask sensitive questions through patient-centered interviewing techniques. This style of interviewing encourages the patient to lead the discussion, allowing a therapeutic bond to develop between the patient and the HCP.31,32 Effective communication relies on concepts such as active listening, empathy, acceptance of the patient’s reality and understanding their agenda, use of an open questioning style, being attuned to nonverbal messages, setting realistic goals, providing education and reassurance, and empowering the patient.26 Allowing the patient a few minutes to describe their symptoms and to discuss their impact is a practical use of time in both the diagnosis and management of IBS.
Recognizing IBS
In a survey of patients with IBS, the most common symptoms resulting in consultation with an HCP were abdominal pain and altered bowel habits (constipation or loose stools).6 In addition to these primary symptoms, patients may also experience bloating, fecal urgency and incontinence, or straining and feelings of incomplete evacuation.8,33,34
If IBS is suspected, it is important to conduct a careful and thorough assessment of the patient’s medical history and a physical examination to determine whether the presentation is consistent with a diagnosis of IBS based on clinical criteria1,8 (Figure 2). Symptom history should be evaluated, including the most frequent or bothersome symptoms and symptom impact, as well as dietary habits and potential symptom triggers. Comorbidities should be assessed, including other chronic GI tract disorders such as celiac disease or inflammatory bowel disease, non-GI tract disorders such as fibromyalgia, psychiatric comorbidities, and other medical conditions such as thyroid disorders or neurologic disease. A thorough evaluation of a patient’s medication list should be undertaken to rule out any possible adverse effects that could cause or contribute to their symptoms. Patients should be asked whether they have undergone any prior evaluations by other HCPs, including gastroenterologists, and, if so, the results of these evaluations. In addition, patients should be asked about any prior medications or other treatment interventions, and, if applicable, their response to the treatment. The patient’s psychosocial history should also be discussed, such as any prior history of abuse or psychological distress, and any interference with work and activities. In addition, patient goals and expectations should be ascertained. Finally, a physical examination should consist of examination of the abdomen, rectum, and pelvis.35,36 This examination is primarily to rule out organic disease; in patients with IBS, the findings are usually normal. There may be abdominal tenderness and tympany (hollow sound), but severe abdominal tenderness, abdominal masses, organomegaly, and/or blood in the stool should prompt evaluation for organic disease.
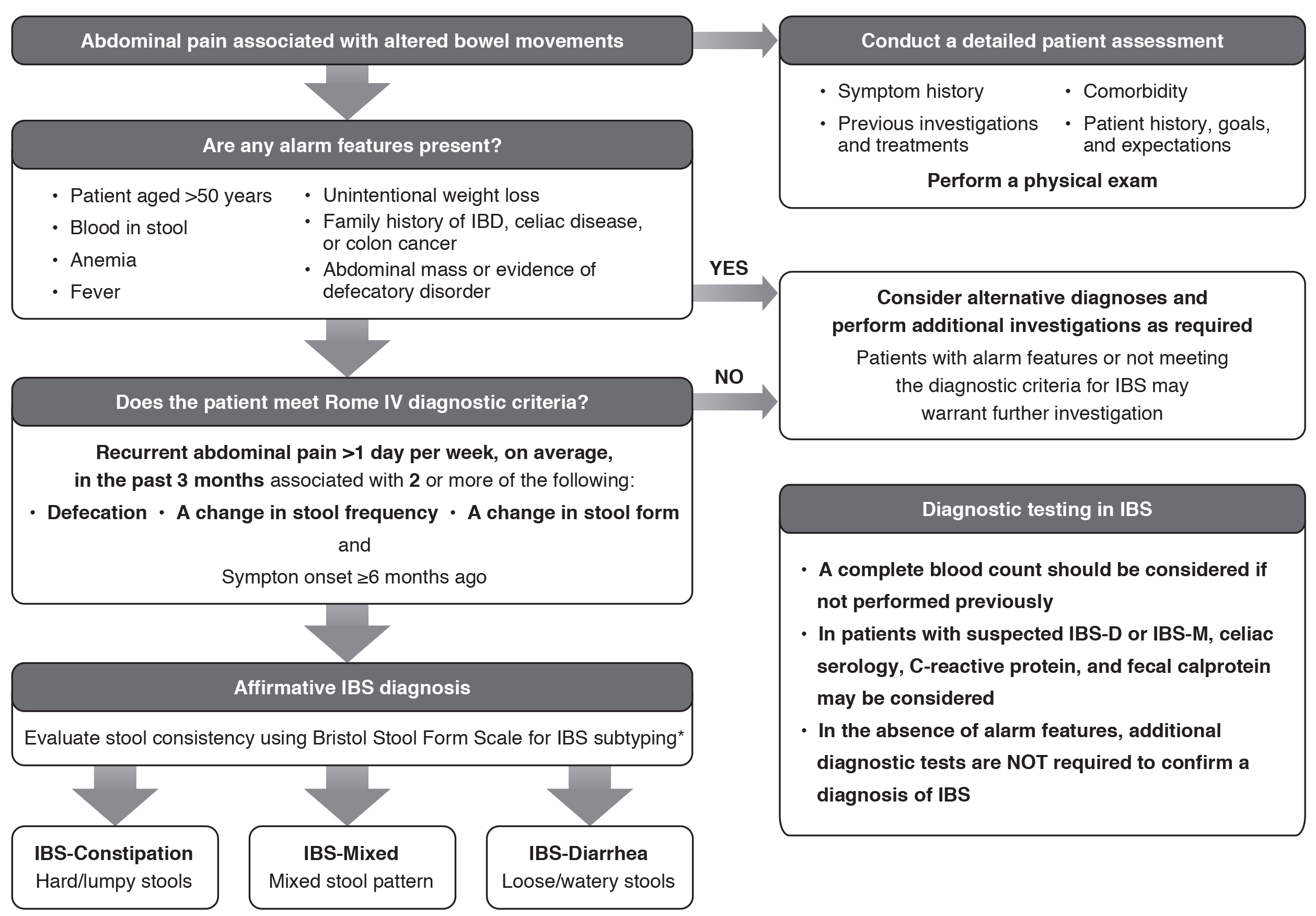
Figure 2. Diagnostic Pathway for IBS. *Patients who meet the diagnostic criteria for IBS but whose bowel habits cannot be accurately categorized into 1 of these 3 groups should be categorized as IBS-Unclassified. Abbreviations: IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome with diarrhea;
IBS-M, irritable bowel syndrome with a mixed stool pattern.
Alarm Features, Diagnostic Testing, and Differential Diagnosis
The presence of alarm features should be assessed to rule out the possibility of organic disease1,8,37 (Figure 2). In patients with alarm features or those who do not meet the diagnostic criteria for IBS, differential diagnoses should be considered and further assessments carried out as required3,8,13,37 (Table 1).
Table 1. Alarm Features to Be Aware of in Patients With Potential or Confirmed IBS | |
Alarm Features | Potential Differential Diagnosis |
Anemia | Celiac disease, colon cancer |
Family history of colorectal cancer, IBD, or celiac disease | Celiac disease, IBD, colon cancer |
Nocturnal diarrhea that awakens the patient | Colorectal cancer, IBD, microscopic colitis |
Onset at age >50 years | Colon cancer, microscopic colitis |
Recent antibiotic use | Clostridium difficile colitis |
Rectal bleeding | IBD, colon cancer, hemorrhoids, ischemic colitis |
Unintentional weight loss (>10% of body weight) | Ischemic colitis, IBD, celiac disease, colon cancer |
Persistent frequent diarrhea without hematochezia | Bile acid malabsorption |
Persistent bloating and diarrhea unresponsive to dietary interventions | Small intestinal bacterial overgrowth |
Abbreviations: IBD, inflammatory bowel disease; IBS, irritable bowel syndrome. | |
In the absence of alarm features, further diagnostic testing is not required to make a confident diagnosis of IBS.8,37,39 However, in certain situations, limited testing may be appropriate as part of the diagnosis1,8 (Table 2). For patients who have had GI tract symptoms that have remained unchanged for many years, further targeted workup can be performed.
Table 2. Recommendations for Diagnostic Testing in IBS1,8 | |
Test | Recommendation |
Complete blood cell count | Consider if not previously performed |
Serum chemistries | Not recommended in patients with typical IBS symptoms and no alarm signs |
Serologic screening for celiac disease | Patients with possible IBS-D or IBS-M |
Small-intestinal bacterial overgrowth breath test | Insufficient data to recommend |
Routine colonoscopy | Not recommended in patients age <50 years without alarm signs |
C-reactive protein and fecal calprotectin | Perform if IBD is considered in the differential diagnosis for patients with potential IBS-D or IBS-M |
Abbreviations: IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with a mixed stool pattern. | |
Colonoscopy is generally not required to diagnose IBS and is not recommended in the absence of alarm features. It has also been shown that negative findings on colonoscopy do not result in reassurance or improved quality of life in patients with IBS.40 Patients may be concerned about an increased cancer risk, but colorectal cancer is no more common in patients with IBS than in the general population.1,39 However, if the patient is over age 50 years or if alarm signs are detected, it may be necessary to refer the patient for a colonoscopy and colon biopsy to assess for colon cancer, microscopic colitis, or inflammatory conditions.16 If celiac disease is suspected, an upper endoscopy with duodenal biopsy in addition to serologic testing should be considered.
Making a Confident Diagnosis of IBS
IBS is not a diagnosis of exclusion; a diagnosis can be established using the criteria developed by the Rome Foundation, a not-for-profit organization that provides support for activities to assist in the diagnosis and treatment of GI tract disorders. The Rome criteria are validated and have a 98% positive predictive value for IBS.8,41 Diagnosis of IBS requires the patient to report recurrent abdominal pain for an average of at least 1 day per week over the past 3 months, and this pain must be associated with 2 or more of the following: defecation, a change in stool frequency, and a change in stool form. In addition, these symptoms must have begun at least 6 months ago8 (Figure 2), and there should be an absence of alarm signs (Table 1).
The Bristol Stool Form Scale is a validated descriptor of stool form and consistency that correlates well with intestinal transit time and is a useful tool to determine IBS subtype in line with the Rome criteria8,42 (Figure 3). Once a diagnosis of IBS is made, the IBS subtype classification may change over time within an individual patient, and symptom severity may fluctuate.43

*Patients who meet the diagnostic criteria for IBS but whose bowel habits cannot be accurately categorized into 1 of these 3 groups should be categorized as IBS-Unclassified.
Abbreviations: IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with a mixed stool pattern.
Modified from original version, ©2006 Rome Foundation. Used with permission.
When to Refer
In most cases, IBS can be successfully diagnosed and managed in the primary care setting; however, referral may be required in certain situations, including on patient request. Table 3 presents situations in which referral of a patient may be required, such as diagnostic uncertainty, the presence of alarm signs or certain comorbidities, or failed response to initial treatment.
Table 3. Situations When Referral to Another Specialist May Be Considered | |
Situation Potentially Requiring Referral | Whom to Refer to |
Diagnosis of IBS is uncertain | Gastroenterologist |
Patient displays atypical presentation | Gastroenterologist |
Patient presents with alarm features (at diagnosis or subsequent to diagnosis)a | Gastroenterologist |
Patient has unintentional weight loss of >5% of body weight within 6 weeks or >10% within 6 months* | Gastroenterologist, dietitian |
Patient has psychological distress or psychiatric comorbidity | Mental health professional |
Patient displays problematic eating behaviors, such as binge eating or overly rigid thought patterns regarding consumption of unhealthy foods | Dietitian |
Patient has extensive questions about food and meal planning that exceed appointment times and interfere with proper medical assessment | Dietitian |
Patient does not show a response to simple diet and lifestyle modifications | Dietitian, gastroenterologist |
Patient does not show a response to pharmacological therapy after sustained treatment attempts | Mental health professional, gastroenterologist |
*Patients displaying alarm signs should always be referred to a gastroenterologist for further investigation. | |
Managing IBS
The overall aims are to help patients effectively manage their IBS symptoms and to improve their disease-related quality of life. Understanding the needs of the patient, setting realistic expectations, and providing further reassurance and education where needed are important.1,26 Once a diagnosis is established, validating the experience of the patient and their symptoms is vital; subsequent reassurance, such as affirming that IBS is not associated with an increased risk of cancer or colitis and does not cause malnutrition or lead to a reduced lifespan, is also important.28,29 Encouraging the patient to become mindful of the association between symptoms and activities, diet, and stress creates a partnership and facilitates progress toward symptom amelioration. A considerable placebo response has been observed in controlled trials in IBS, and it has been shown that the patient–HCP relationship provides the largest contribution to this effect.45 NPs and PAs are uniquely qualified to spend time with patients and establish a positive, communicative, and therapeutic relationship. While IBS cannot be cured, it can be effectively managed in most cases. Due to the diverse underlying pathophysiological processes, effective management of IBS is multifactorial, combining pharmacological and nonpharmacological therapies with dietary and lifestyle modifications, and should be based on IBS subtype, patient preference, and symptom severity.1,8,46
Patients with IBS should be followed up regularly to assess therapeutic response and the development of any alarm signs (Table 1). Follow-up every 2 weeks to 6 months is generally suitable, dependent on patient symptoms, history, expectations, and treatment goals. More regular follow-up intervals may be beneficial when initiating and refining a treatment plan and to solidify patient-led lifestyle modifications. If a patient’s symptoms do not improve with initial treatment, additional or alternative treatment options or referral should be considered.34 Maintaining regular communication with any other specialists involved in patient management, such as gastroenterologists, psychotherapists, and dietitians, is also important to ensure optimal management.
Lifestyle Modifications
Lifestyle modifications, such as an increase in physical activity, improvements in sleep hygiene, and reduction in anxiety and stress, are a mainstay of IBS management. However, for some patients, the severity of their symptoms or implementation of the lifestyle changes required may be a barrier to symptom control. As with any lifestyle modification, setting mutually agreeable goals with the patient and family may increase adherence. Success is more likely if the patient sets the goals in conjunction with the NP or PA, with the following evidence-based modifications being recommended.
Patients with IBS should be encouraged to become more physically active, for example taking a 20-minute walk each day with distance and pace being gradually increased as tolerated, since exercise stimulates GI motility and can improve colonic transit.1,47 Many patients with IBS experience worsening of symptoms with stress and anxiety, so stress-reduction techniques such as meditation, yoga, and deep breathing may be beneficial.48,49
IBS can also result in sleep disturbance that in turn worsens symptoms and quality of life.50,51 Sleep hygiene can be improved through measures such as relaxation, avoiding caffeine, and limiting screen time before bed. Patients should be encouraged to sleep in a room that is cool and dark, ideally without LEDs or small electronic lights; if present, these should be covered at bedtime.
Diet
Although food allergies are no more common in patients with IBS than in the general population, worsening of GI tract symptoms following meals and adverse reactions to certain food items are commonly experienced by patients, with approximately two-thirds considering their symptoms to be related to meals.17,18,52 As a result, most patients with IBS will alter their eating habits, although responses to specific foods generally have not been evaluated in a rigorous fashion.53-56
Open-ended, diet-related questions such as whether specific meals or foods worsen symptoms, whether the patient has any “go-to” foods for when they are trying to avoid symptoms, and whether they think sleep and alcohol play a role in their symptoms can help to uncover dietary patterns and any symptom triggers. General dietary guidance can be provided at this stage, with an emphasis on regular eating patterns and portion sizes, in order to evaluate whether a predictable symptom pattern can be observed. This information can then inform more-specific dietary or lifestyle guidance as needed.
General dietary guidance. General dietary modifications, such as those provided by the UK National Institute for Health and Care Excellence, may improve IBS symptom severity, including eating regular small meals and increasing fluid intake.57 Reducing intake of coffee, carbonated drinks, alcohol, and milk products can also be of benefit in IBS,54,55 as can eliminating foods known to trigger IBS symptoms in some patients, including fatty and spicy food and certain fruits and vegetables such as beans or lentils, apples, potatoes, bananas, and avocados.17,18 Intake of fiber should be assessed, and soluble fiber intake from sources such as psyllium husk, flax, and fruit pulp can be gradually increased if not optimal, since these can provide relief of IBS symptoms.53,56,58 Avoidance of insoluble fibers such as wheat bran is recommended, since these can exacerbate IBS symptoms.53,55,56,58 Despite the use of probiotics having been evaluated in multiple trials, the evidence supporting it in IBS is mixed, and it is not currently possible to make specific recommendations on individual bacterial species, preparations, or strains due to limited data, although probiotics as a category appear to improve global symptoms, bloating, and flatulence in IBS.53,56,58
It is important to note that dietary recommendations can vary greatly from patient to patient, so it is essential to develop an individualized plan based on patient symptoms and reported triggers. Referral to a registered dietitian may be warranted in certain cases.
Low-FODMAP diet. Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are poorly absorbed carbohydrates found in foods that act as substrates for gas production and bacterial fermentation. Common low- and high-FODMAP foods are summarized in Figure 4.59 A diet low in FODMAPs is associated with improvement in IBS symptoms,52,60-62 and approximately half of patients with IBS administered a low-FODMAP diet reported a possible benefit in their overall symptoms.56 However, a low-FODMAP diet should be carried out under the supervision of a registered dietitian due to the required expertise on dietary sources of FODMAPs and to avoid deficiencies in fiber, vitamins, and minerals.55 In addition, the diet should not be undertaken long term; gradual reintroduction of foods after 6 to 8 weeks should be encouraged.52,55

Abbreviation: FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Gluten-free diet. The prevalence of celiac disease among patients with IBS-D is similar to that of persons without IBS.63 However, nonceliac gluten sensitivity may occur in a subset of patients with IBS, and a trial of a gluten-free diet under supervision of a registered dietitian may be of benefit in patients with IBS in whom general dietary modifications have not been effective.63,64 It should be noted that the American College of Gastroenterology suggests against a gluten-free diet for overall symptom improvements in IBS patients due to a current lack of evidence in the form of randomized controlled trials.56 The association between gluten exposure and IBS is not yet well understood, since wheat also contains fructans and other proteins that may cause symptoms.1,63 In addition, there are currently no defined predictors of which patients with IBS will respond to gluten elimination.64
Psychological Therapies
Because the pathogenesis of IBS involves both physiological and psychological factors that interact in a bidirectional fashion between the gut and the brain, psychotherapy strategies may be of benefit in IBS, including cognitive behavioral therapy (CBT), gut-directed hypnotherapy, psychodynamic (interpersonal) therapy (PIT), and mindfulness-based therapy (MBT).16,46
Of the available psychological therapies for IBS, the strongest body of evidence exists to support CBT, which can improve IBS symptoms, quality of life, and psychological comorbidities.16,53,56 CBT aims to educate patients about physical, cognitive, and behavioral factors that contribute to IBS in order to enhance self-control of symptoms and prevent symptom relapse. Patients learn to modify behaviors, correct dysfunctional thoughts, and control stress and anxiety, which can all contribute to IBS symptoms.16,65 CBT requires a significant amount of patient time, regular provider visits, and the availability of a trained therapist.65
In gut-directed hypnotherapy, a trance or relaxed state is induced, and suggestions are made on how to control and normalize GI function. Multiple sessions may be required, and patients are provided with information and encouraged to practice at home on a daily basis.16,46,66 Research to identify predictors of response to hypnotherapy has been inconclusive, and the availability of experienced, skilled practitioners may be limiting.66
The validation and body of evidence for PIT and MBT in IBS are currently more limited compared with CBT.16,53,67 PIT involves exploration of links between symptom development and interpersonal conflicts or relationship difficulties, with an in-depth discussion of symptoms and exploration of emotional factors, leading to reductions in psychological distress.16,67,68 MBT in IBS is a group program that focuses on patients’ perceptions of symptoms and coping strategies and may reduce IBS symptom severity; however, current supportive evidence is limited.16,67,69
Pharmacological Interventions
If lifestyle or dietary modifications are unsuccessful in improving IBS symptoms, it is appropriate to consider initiation of pharmacological therapy, although any lifestyle modifications should also be continued throughout treatment as part of a multifactorial management approach. Medication selection is dependent on IBS subtype and the patient’s most bothersome symptom, such as altered bowel habit or abdominal pain. Other factors influencing the choice of medication are disease severity, response to any prior medications used, and patient and provider preferences. Often more than one pharmacological agent may be used in combination, such as gut-acting and centrally acting agents.
Once a medication is initiated, it is important to assess response and for possible adverse events after 2 to 4 weeks. If an insufficient response is seen at follow-up, the dose can be adjusted, treatments can be combined to address other bothersome IBS symptoms, including addition of psychological and psychopharmacological interventions, or treatment can be escalated to another option.
Nonprescription Medications
Most patients with IBS report having tried an over-the-counter (OTC) medication before consulting with an HCP, with an average of 3.6 OTC medications being trialed.6 Several OTC medication options are commonly used in the management of IBS (Table 4). Individual OTC medications may address specific symptoms of IBS but in general do not address the multiple symptoms seen in IBS or the underlying pathophysiology.15,34,53,56,58,70
Although OTC treatments are widely used, there is limited evidence that they effectively address the broad range of abdominal and bowel symptoms experienced by patients, and patient satisfaction with these treatments can be low.6,34,53,56,58,70 Inadequate symptom control in IBS has been shown to result in increased health care resource use and increased direct costs.90,91
Table 4. Overview of Medications Commonly Used in the Management of IBS | |||||
| Mechanism of Action | Indication | Dose and Administration | Efficacy—Key Points to Consider | Safety—Key Points to Consider |
OTC medications | |||||
Peppermint oil | Smooth-muscle relaxant | Available OTC | 1-2 capsules TID orally, 15-30 minutes before food for 1 month34 | • Improves abdominal pain, discomfort, and bloating58 | • Most common AE is heartburn |
Loperamide | Antidiarrheal | Available OTC | No known optimal dose; 2-8 mg/d used in IBS-D trials34 | • Improves stool consistency and frequency in IBS-D53 | • Common AEs include constipation, nausea, abdominal cramping, and dizziness34 |
Polyethylene glycol | Osmotic laxative | Available OTC | 17 g/d, dissolved in 8 oz water72,73 | • Improves stool consistency and frequency in IBS-C58 | • Common AEs include abdominal pain and headache53 |
FDA-approved medications for the treatment of IBS | |||||
IBS-D | |||||
Alosetron | 5-HT antagonist | Treatment of women with severe IBS-D who have not responded adequately to conventional therapy74 | 0.5 mg BID74 | • Global relief of IBS symptoms, diarrhea, and abdominal pain in phase 3 trials58 | • Reports of infrequent but serious AEs including ischemic colitis and serious consequences of constipation, resulting in hospitalization and, rarely, blood transfusion, surgery, and death74 |
Eluxadoline | Opioid receptor ligand | Treatment of IBS-D in adults75 | 100 mg BID orally with food75 | • Simultaneously improved abdominal pain and stool consistency and improved global symptoms in phase 3 trials76 | • Common AEs include nausea, constipation, and abdominal pain76 |
Rifaximin | Broad-spectrum antibiotic | Treatment of IBS-D in adults80 | 550 mg TID orally for 14 days80 | • Adequate relief of global symptoms and improvements in bloating, abdominal pain, and stool consistency in phase 3 trials81 | • Common AEs include headache, upper respiratory tract infection, and abdominal pain81 |
IBS-C | |||||
Linaclotide | Guanylate cyclase-C agonist | Treatment of IBS-C in adults83 | 290 µg QD orally83 | • Simultaneously improved abdominal pain and number of CSBMs and improved bloating and stool consistency in phase 3 trials84,85 | • Common AEs include diarrhea, abdominal pain, and flatulence84,85 |
Lubiprostone | Chloride channel activator | Treatment of IBS-C in adult women86 | 8 µg BID orally with food and water86 | • Provided relief of IBS symptoms and improved abdominal pain and stool consistency in phase 3 trials53,87 | • Common AEs include diarrhea and nausea53 |
Plecanatide | Guanylate cyclase-C agonist | Treatment of IBS-C in adults88 | 3 mg QD orally88 | • Simultaneously improved abdominal pain and number of CSBMs88,89 | • Most common AE is diarrhea88 |
Non-FDA-approved prescription medications used in IBS in clinical practice | |||||
Antispasmodics: Hyoscyamine, dicyclomine | Smooth-muscle relaxant | Not approved for use in IBS | Hyoscyamine, up to 1.5 mg/d; Dicyclomine, 20-40 mg QD34 | • Can provide short-term symptom relief58 | • Common AEs include dry mouth, dizziness, and blurred vision53 |
SSRIs: Fluoxetine, paroxetine, citalopram | Serotonin reuptake inhibitor | Not approved for use in IBS | Fluoxetine, 20 mg QD; paroxetine, 10-50 mg QD; citalopram, 20-40 mg QD34 | • Effective in providing global symptom relief and improving pain53 | • Common AEs include nausea, insomnia, diarrhea or constipation, decreased libido, ejaculatory dysfunction, and weight gain34 |
TCAs: Amitriptyline, desipramine, trimipramine, imipramine, doxepin | Not fully understood | Not approved for use in IBS | 10-150 mg/d, depending on drug; generally >50 mg/d34 | • Effective in providing global symptom relief and improving pain53 | • Common AEs include dry mouth, constipation, blurred vision, urinary retention, sedation, weight gain, sexual dysfunction, arrhythmia, dizziness, and hyperthermia34 |
Abbreviations: AE, adverse event; BID, twice daily; CSBM, complete spontaneous bowel movement; FDA, Food and Drug Administration; | |||||
Medications Approved by the Food and Drug Administration (FDA) for IBS
Several prescription medications are now FDA-approved for the treatment of IBS and may be suitable in patients whose symptoms are more severe or not adequately controlled by lifestyle interventions or other medication options.3,34,53,58,70 The choice of therapy can be based on the primary presenting symptom, whether that be diarrhea, constipation, or abdominal pain. Prescription medication options include alosetron, eluxadoline, and rifaximin in patients with IBS-D and linaclotide, lubiprostone, and plecanatide in patients with IBS-C (Table 4).
Prescription Medications Not FDA-Approved for IBS
Although not approved by the FDA for the treatment of IBS, certain prescription medications such as antidepressants and antispasmodics are used off-label (Table 4). Antidepressants are often used, with tricyclic antidepressants being recommended for overall symptom improvement in patients with IBS. The evidence for the use of selective serotonin-reuptake inhibitors is weak; therefore, they are suggested but not recommended by the American College of Gastroenterology.56 The evidence for the use of antispasmodics is also weak56; however, they are sometimes prescribed in patients with abdominal cramping, although they may cause constipation and so should be used with caution in patients with IBS-C.34,53,58,70 Patient and HCP acceptance and associated adverse events may also limit the utility of these options for the management of IBS.53
Conclusion
IBS is one of the most common GI tract disorders and causes a significant impact on patients’ lives. Due to their role in the health care system and their position on the front line of day-to-day patient management, particularly those with chronic symptoms, NPs and PAs are in a unique position to cultivate a therapeutic, trusting, and communicative relationship with patients with IBS and provide continuity of care with close follow-up. A diagnosis of IBS can be efficiently established based on symptoms and the exclusion of alarm features, with minimal diagnostic testing required. Management of IBS is multifaceted and should be tailored to the patient, potentially involving lifestyle interventions and dietary modifications in addition to escalating pharmacological therapies, depending on the predominant symptom, symptom severity, impact on daily life, and presence of comorbidities. The formation of an effective patient–provider relationship is key to the successful management of this common GI tract disorder.
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313(9):949-958.
- Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ III. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101(4):927-934.
- Lacy BE, Moreau JC. Diarrhea-predominant irritable bowel syndrome: diagnosis, etiology, and new treatment considerations. J Am Assoc Nurse Pract. 2016;28(7):393-404.
- Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
- Saito YA, Schoenfeld P, Locke GR III. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910-1915.
- American Gastroenterological Association. IBS in America: survey summary findings. http://www.multivu.com/players/English/7634451-aga-ibs-in-america-survey/docs/survey-findings-pdf-635473172.pdf. December 2015. Accessed November 27, 2018.
- Hungin APS, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21(11):1365-1375.
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393-1407.e5.
- El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16(6):1171-1185.
- Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004;10(4):299-309.
- Buono JL, Mathur K, Averitt AJ, Andrae DA. Economic burden of irritable bowel syndrome with diarrhea: retrospective analysis of a U.S. commercially insured population. J Manag Care Spec Pharm. 2017;23(4):453-460.
- Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: a multivariable analysis. Gastroenterology. 2004;126(7):1665-1673.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491.
- Sayuk GS, Wolf R, Chang L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2017;112(6):892-899.
- Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133-146.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014.
- Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634-641.
- Simrén M, Månsson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108-115.
- Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33(6):825-830.
- Ballou S, Bedell A, Keefer L. Psychosocial impact of irritable bowel syndrome: a brief review. World J Gastrointest Pathophysiol. 2015;6(4):120-123.
- Fadgyas-Stanculete M, Buga A-M, Popa-Wagner A, Dumitrascu DL. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J Mol Psychiatry. 2014;2(1):4.
- Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64(6):573-582.
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140-1156.
- Weinstock LB, Walters AS. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med. 2011;12(6):610-613.
- Taft TH, Riehl ME, Dowjotas KL, Keefer L. Moving beyond perceptions: internalized stigma in the irritable bowel syndrome. Neurogastroenterol Motil. 2014;26(7):1026-1035.
- Drossman DA. 2012 David Sun lecture: helping your patient by helping yourself—how to improve the patient–physician relationship by optimizing communication skills. Am J Gastroenterol. 2013;108(4):521-528.
- Taft TH, Keefer L, Artz C, Bratten J, Jones MP. Perceptions of illness stigma in patients with inflammatory bowel disease and irritable bowel syndrome. Qual Life Res. 2011;20(9):1391-1399.
- Halpert A, Dalton CB, Palsson O, et al. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National survey on patient educational needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). Am J Gastroenterol. 2007;102(9):1972-1982.
- Lacy BE, Weiser K, Noddin L, et al. Irritable bowel syndrome: patients’ attitudes, concerns and level of knowledge. Aliment Pharmacol Ther. 2007;25(11):1329-1341.
- Lacy BE, Chey WD, Cash BD, Lembo AJ, Dove LS, Covington PS. Eluxadoline efficacy in IBS-D patients who report prior loperamide use. Am J Gastroenterol. 2017;112(6):924-932.
- Lyles JS, Dwamena FC, Lein C, Smith RC. Evidence-based patient-centered interviewing. J Clin Outcomes Manag. 2001;8(7):28-34.
- Platt FW, Gaspar DL, Coulehan JL, et al. “Tell me about yourself”: the patient-centered interview. Ann Intern Med. 2001;134(11):1079-1085.
- Atarodi S, Rafieian S, Whorwell PJ. Faecal incontinence—the hidden scourge of irritable bowel syndrome: a cross-sectional study. BMJ Open Gastroenterol. 2014;1(1):e000002.
- Lucak S, Chang L, Halpert A, Harris LA. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: evidence-based treatment in practice. Therap Adv Gastroenterol. 2017;10(2):253-275.
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108-2131.
- Lucak S. Diagnosing irritable bowel syndrome: what’s too much, what’s enough? MedGenMed. 2004;6(1):17.
- American College of Gastroenterology IBS Task Force. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(suppl 1):S1-S35.
- Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7-17.
- Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97(11):2812-2819.
- Spiegel BMR, Gralnek IM, Bolus R, et al. Is a negative colonoscopy associated with reassurance or improved health-related quality of life in irritable bowel syndrome? Gastrointest Endosc. 2005;62(6):892-899.
- Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94(10):2912-2917.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920-924.
- Garrigues V, Mearin F, Badía X, et al; RITMO Group. Change over time of bowel habit in irritable bowel syndrome: a prospective, observational, 1-year follow-up study (RITMO study). Aliment Pharmacol Ther. 2007;25(3):323-332.
- O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300(6722):439-440.
- Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999-1003.
- Eriksson EM, Andrén KI, Kurlberg GK, Eriksson HT. Aspects of the non-pharmacological treatment of irritable bowel syndrome. World J Gastroenterol. 2015;21(40):11439-11449.
- Asare F, Störsrud S, Simrén M. Meditation over medication for irritable bowel syndrome? On exercise and alternative treatments for irritable bowel syndrome. Curr Gastroenterol Rep. 2012;14(4):283-289.
- Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592-605.
- Park S-H, Han KS, Kang C-B. Relaxation therapy for irritable bowel syndrome: a systematic review. Asian Nurs Res. 2014;8(3):182-192.
- Patel A, Hasak S, Cassell B, et al. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(3):246-258.
- International Foundation for Functional Gastrointestinal Disorders. Sleep and irritable bowel syndrome. https://www.aboutibs.org/signs-and-symptoms-main/sleep-and-irritable-bowel-syndrome-2.html. Updated July 6, 2016. Accessed November 27, 2018.
- DeWeerdt S. Diet: food for thought. Nature. 2016;533(7603):S108-S109.
- Ford AC, Moayyedi P, Lacy BE, et al; Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
- Hayes PA, Fraher MH, Quigley EMM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y). 2014;10(3):164-174.
- McKenzie YA, Bowyer RK, Leach H, et al; IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):549-575.
- Ford AC, Moayyedi P, Chey WD, et al; ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(suppl 2):1-18.
- National Institute for Health and Care Excellence. Irritable bowel syndrome in adults: diagnosis and management. https://www.nice.org.uk/guidance/cg61/resources/irritable-bowel-syndrome-in-adults-diagnosis-and-management-pdf-975562917829. Published February 23, 2008. Accessed November 27, 2018.
- Chey WD. Symposium report: an evidence-based approach to IBS and CIC: applying new advances to daily practice: a review of an adjunct clinical symposium of the American College of Gastroenterology Meeting October 16, 2016, Las Vegas, Nevada. Gastroenterol Hepatol (N Y). 2017;13(2 suppl 1):1-16.
- Cozma-Petruţ A, Loghin F, Miere D, Dumitraşcu DL. Diet in irritable bowel syndrome: what to recommend, not what to forbid to patients! World J Gastroenterol. 2017;23(21):3771-3783.
- Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399-1407.
- Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6(7):765-771.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25(2):252-258.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903-911.
- Makharia A, Catassi C, Makharia GK. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: a clinical dilemma. Nutrients. 2015;7(12):10417-10426.
- Tang Q-L, Lin G-Y, Zhang M-Q. Cognitive-behavioral therapy for the management of irritable bowel syndrome. World J Gastroenterol. 2013;19(46):8605-8610.
- Peters SL, Muir JG, Gibson PR. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(11):1104-1115.
- Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: a systematic review and meta-analysis. Clin Psychol Rev. 2017;51:142-152.
- Hyphantis T, Guthrie E, Tomenson B, Creed F. Psychodynamic interpersonal therapy and improvement in interpersonal difficulties in people with severe irritable bowel syndrome. Pain. 2009;145(1-2):196-203.
- Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106(9):1678-1688.
- Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion. 2014;89(4):253-267.
- Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61(2):560-571.
- DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MvB. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446-450.
- DiPalma JA, Cleveland MvB, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102(7):1436-1441.
- Lotronex [highlights of prescribing information]. San Diego, CA: Prometheus Laboratories Inc; 2014. https://www.lotronex.com/hcp/_docs/Lotronex_PI.pdf. Accessed November 27, 2018.
- Viberzi [highlights of prescribing information]. Cincinnati, OH: Forest Pharmaceuticals Inc; 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206940s000lbl.pdf. Accessed November 27, 2018.
- Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374(3):242-253.
- Drug Enforcement Administration Diversion Control Division. Mid-level practitioners authorization by state. https://www.deadiversion.usdoj.gov/drugreg/practioners/mlp_by_state.pdf. Accessed November 27, 2018.
- Levy-Cooperman N, McIntyre G, Bonifacio L, et al. Abuse potential and pharmacodynamic characteristics of oral and intranasal eluxadoline, a mixed µ- and κ-opioid receptor agonist and δ-opioid receptor antagonist. J Pharmacol Exp Ther. 2016;359(3):471-481.
- Fant RV, Henningfield JE, Cash BD, Dove LS, Covington PS. Eluxadoline demonstrates a lack of abuse potential in phase 2 and 3 studies of patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2017;15(7):1021-1029.
- Xifaxan [highlights of prescribing information]. Raleigh, NC: Salix Pharmaceuticals Inc; 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021361s012lbledt.pdf. Accessed November 27, 2018.
- Pimentel M, Lembo A, Chey WD, et al: TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22-32.
- Pimentel M, Cash BD, Lembo A, Wolf RA, Israel RJ, Schoenfeld P. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci. 2017;62(9):2455-2463.
- Linzess [highlights of prescribing information]. Irvine, CA: Allergan USA Inc; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202811s013lbl.pdf. Accessed November 27, 2018.
- Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107(11):1702-1712.
- Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714-1724.
- Amitiza [highlights of prescribing information]. Bethesda, MD: Sucampo Pharma Americas Inc; 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021908s010lbl.pdf. Accessed November 27, 2018.
- Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29(3):329-341.
- Trulance [highlights of prescribing information]. New York, NY: Synergy Pharmaceuticals Inc; 2018. https://www.trulance.com/prescribing-information.pdf. Accessed November 27, 2018.
- Miner PB Jr. Efficacy and safety of plecanatide in treating constipation predominant irritable bowel syndrome. Expert Opin Pharmacother. 2018;19(2):177-183.
- Buono JL, Mathur K, Averitt AJ, Andrae DA. Economic burden of inadequate symptom control among US commercially insured patients with irritable bowel syndrome with diarrhea. J Med Econ. 2017;20(4):353-362.
- Guerin A, Carson RT, Lewis B, Yin D, Kaminsky M, Wu E. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J Med Econ. 2014;17(8):577-586.


