A Tale of Two "Pulseless Electrical Activity" Cardiac Arrest Rhythms

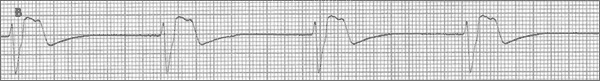 Case 1: A 46-year-old man with melena is admitted to the ICU. Cardiac arrest occurs while he awaits an upper esophagogastroduodenoscopy; he immediately becomes unresponsive, apneic, and pulseless. The ECG monitor shows a rapid, narrow-QRS-complex tachycardia with obvious P-wave activity, which in this case represents pulseless electrical activity (PEA) (Figure 1 [A]). The patient's hematocrit on admission was 39% (with a healthy baseline value of 46%); the hematocrit just before the arrest was 31%. Treatment is started with attention to the airway, breathing, and circulation. In addition to standard advanced life support management, normal saline is rapidly infused via a central venous line followed by 4 units of packed red blood cells. Resuscitation continues.
Case 1: A 46-year-old man with melena is admitted to the ICU. Cardiac arrest occurs while he awaits an upper esophagogastroduodenoscopy; he immediately becomes unresponsive, apneic, and pulseless. The ECG monitor shows a rapid, narrow-QRS-complex tachycardia with obvious P-wave activity, which in this case represents pulseless electrical activity (PEA) (Figure 1 [A]). The patient's hematocrit on admission was 39% (with a healthy baseline value of 46%); the hematocrit just before the arrest was 31%. Treatment is started with attention to the airway, breathing, and circulation. In addition to standard advanced life support management, normal saline is rapidly infused via a central venous line followed by 4 units of packed red blood cells. Resuscitation continues.
Case 2: A 76-year-old woman with cardiac arrest is brought to the emergency department (ED) via ambulance. At home, she was found unresponsive, pulseless, and apneic. She has a history of myocardial infarction (MI); congestive heart failure, with an ejection fraction of 0.15; and chronic renal failure. Emergency medical personnel initiated appropriate care for ventricular fibrillation (VF), including cardiopulmonary resuscitation, airway management, defibrillation, intravenous medications, and intravenous fluids. After defibrillation, the post-countershock rhythm was a wide-QRS-complex, regular rhythm without pulse (Figure 1 [B]), which persisted at the time of ED arrival. Total downtime was approximately 20 minutes. Advanced life support care is continued.
Both these patients exhibit PEA cardiac arrest rhythms. Which statement about such patients is not true?
A. The cardiac rhythm can be used to guide therapy in resuscitation.B. "True" and "pseudo" subtypes of PEA have similar causes and outcomes.
C. A rapid, narrow-QRS-complex rhythm is associated with an improved chance of survival.
D. A slow, wide-QRS-complex rhythm is associated with little chance of survival.
CORRECT ANSWER: B
Pulseless electrical activity (PEA) is a malignant dysrhythmia that reflects a serious underlying medical event. PEA is characterized by the unique combination of an absence of discernible cardiac mechanical activity (ie, a "pulseless" state) with persistent cardiac electrical activity (ie, the cardiac rhythm). Essentially, any cardiac arrest rhythm (ie, any dysrhythmia unaccompanied by a pulse) other than ventricular tachycardia or VF may be seen in a patient with PEA.
"TRUE" VERSUS "PSEUDO" PEA
It is important to distinguish between the "true" and "pseudo" subtypes of PEA. In true PEA, cardiac electrical activity in the form of a rhythm is noted, yet absolutely no mechanical contraction of the heart is occurring--hence, the absence of pulse and perfusion. Pseudo PEA, which occurs when cardiac electrical activity is demonstrated but a palpable pulse is absent, is diagnosed when myocardial contractions are noted via echocardiography or some other imaging modality. In patients with pseudo PEA, the ability of the cardiovascular system to generate "forward flow" is impeded despite the continued presence of myocardial contractions.
True PEA. This occurs when there is a primary electromechanical uncoupling of the myocytes--a complete dissociation of electrical depolarization from mechanical cardiac contractions. This uncoupling event is usually characterized by abnormal automaticity and disrupted cardiac conduction--which results in the continued presence of a cardiac rhythm, albeit a slow and/or widened QRS-complex bradycardia. Mechanically, this uncoupling is probably a result of global myocardial energy depletion; local tissue issues (hypoxia, acidosis, hyperkalemia, and ischemia) also contribute to the electromechanical dissociation.
True PEA is seen in settings of prolonged cardiac arrest, as well as in scenarios that involve metabolic derangement, hypothermia, or poisoning. In an important subtype of true PEA, a patient with prolonged VF is defibrillated to a state of electromechanical dissociation with a broad-QRS-complex, bradycardic rhythm. In these patients, near-complete exhaustion of the energy substrate, resulting from profound hypoxia, acidosis, and prolonged arrest, accounts for the dismal rhythm.1,2
Pseudo PEA. In pseudo PEA, a significant pathophysiologic event has impaired the ability of the cardiovascular system to perfuse the body. The event is usually one of the following:
- Profound hypovolemia resulting from hemorrhage.
- Obstruction of forward flow caused by massive pulmonary embolism.
- Tension pneumothorax.
- Cardiac tamponade.
- Hypocontractile states with poor vascular tone, such as advanced anaphylactic or septic shock.
- Very rapid tachydysrhythmias.
In these situations, myocardial contractions continue yet perfusion pressure is very limited, hence the absence of a pulse with continued cardiac electrical activity. Rhythms in these settings are usually tachycardic--predominantly sinus tachycardia or atrial fibrillation with rapid ventricular response.
MANAGEMENT OF PEA: USING CLUES FROM THE RHYTHM
Patients with PEA are invariably gravely ill. The causes of this dysrhythmia are numerous, spanning all of acute care medicine. Acute respiratory illnesses with pre-arrest hypoxia are frequent comorbid conditions.2,3 A number of the causes of PEA are potentially reversible--if they are noted early and correctly treated in the initial phases of resuscitation.
The management of the patient in PEA arrest focuses on both standard resuscitation treatments and the identification and correction of reversible causes. Standard advanced life support measures should include cardiopulmonary resuscitation, provision of an adequate airway, volume expansion, vasopressor therapy, and atropine (if the cardiac rhythm is bradycardic). Additional interventions performed during resuscitation--in particular, pericardiocentesis and needle thoracostomy--have infrequently been associated with return of spontaneous circulation.4
 Like the clinical events that can cause PEA, the rhythms associated with this entity are numerous. The dysrhythmias most frequently seen in PEA include idioventricular, junctional, and sinus bradycardias (Figure 2). The ECG rhythm may be a useful clue to the cause of PEA and thus a guide to appropriate resuscitation measures (Figure 3).
Like the clinical events that can cause PEA, the rhythms associated with this entity are numerous. The dysrhythmias most frequently seen in PEA include idioventricular, junctional, and sinus bradycardias (Figure 2). The ECG rhythm may be a useful clue to the cause of PEA and thus a guide to appropriate resuscitation measures (Figure 3).
 Rapid, narrow-QRS-complex tachycardic presentations usually represent a pseudo PEA cardiac arrest. Profound hypovolemia of various causes (eg, dehydration, hemorrhage) is the most frequently encountered event in patients with PEA, although other events (eg, cardiac tamponade, tension pneumothorax, large pulmonary embolism) can also impair adequate circulation. The heart increases its rate in an attempt to maintain cardiac output as its ability to pump efficiently declines. This increased rate usually manifests as sinus tachycardia; in patients with atrial fibrillation (AF), AF with a rapid ventricular response is seen. In patients with narrow-QRS-complex rhythms, in addition to resuscitative therapies, treatments performed early in the patient's course and aimed at correction of the underlying cause are suggested. Rapid intervention that targets the cause optimizes the likelihood of a good outcome.
Rapid, narrow-QRS-complex tachycardic presentations usually represent a pseudo PEA cardiac arrest. Profound hypovolemia of various causes (eg, dehydration, hemorrhage) is the most frequently encountered event in patients with PEA, although other events (eg, cardiac tamponade, tension pneumothorax, large pulmonary embolism) can also impair adequate circulation. The heart increases its rate in an attempt to maintain cardiac output as its ability to pump efficiently declines. This increased rate usually manifests as sinus tachycardia; in patients with atrial fibrillation (AF), AF with a rapid ventricular response is seen. In patients with narrow-QRS-complex rhythms, in addition to resuscitative therapies, treatments performed early in the patient's course and aimed at correction of the underlying cause are suggested. Rapid intervention that targets the cause optimizes the likelihood of a good outcome.
Conversely, a slow, wide-QRS-complex rhythm is a clue that true PEA is evolving. Prolonged cardiac arrest as well as profound metabolic disarray, severe overdose, hypothermia, end-stage sepsis, and large anterior wall MI are typical clinical scenarios. Slow, wide-complex rhythms are ominous and do not support the need for continued aggressive treatment.
DETERMINING PROGNOSIS
The prognosis of patients with PEA is grim; not surprisingly, the list of associated clinical entities includes numerous malignant syndromes. Thus, the vast majority of patients who present with PEA will not experience a return of spontaneous perfusion or survival to hospital discharge. In fact, only 2% to 4% of patients with PEA as the initial and/or primary dysrhythmia in cardiac arrest survive to hospital discharge with a meaningful quality of post-arrest life.3,5,6 Among patients who survive PEA arrest, mortality approaches 50% in the first 10 days to 6 months after resuscitation; at 1 year, few patients (under 4%) remain alive and well.5
An analysis of the ECG rhythm can be used to prognosticate. In a large prehospital population of patients with PEA cardiac arrest, Aufderheide and colleagues7 noted several ECG variables that predicted successful resuscitation:
- Rapid rates.
- Narrow QRS complexes.
- Development of P waves.
In a similar review of PEA arrest rhythm characteristics, a normal duration QRS complex with P-wave activity was more often encountered in those patients who survived to emergency department admission.8 Thus, sinus tachycardia suggests a markedly better--although still relatively bleak--prognosis than an idioventricular bradycardia. A slow, wide-QRS-complex rhythm predicts a very poor outcome.
OUTCOME OF CASE 1 The patient in Case 1, a middle-aged man with apparent GI hemorrhage and narrow-complex tachycardia PEA, responded rapidly to bolus vasopressor (40 units of vasopressin) and volume expansion with normal saline and blood products. He developed a pulse, with rhythm as shown in Figure 4 (A)--a very rapid sinus tachycardia. Subsequent care revealed an actively bleeding gastric ulcer, which was cauterized. His cardiac arrest had resulted from hemorrhage with profound volume depletion and subsequent PEA. He was discharged from the hospital 2 weeks later with intact neurologic status and minimal personality change.
The patient in Case 1, a middle-aged man with apparent GI hemorrhage and narrow-complex tachycardia PEA, responded rapidly to bolus vasopressor (40 units of vasopressin) and volume expansion with normal saline and blood products. He developed a pulse, with rhythm as shown in Figure 4 (A)--a very rapid sinus tachycardia. Subsequent care revealed an actively bleeding gastric ulcer, which was cauterized. His cardiac arrest had resulted from hemorrhage with profound volume depletion and subsequent PEA. He was discharged from the hospital 2 weeks later with intact neurologic status and minimal personality change.
OUTCOME OF CASE 2 Despite continued aggressive care of the patient in Case 2, the cardiac rhythm demonstrated further widening of the QRS complex with a slowed ventricular rate (Figure 4 [B]). No pulse was noted. Resuscitation was halted 10 minutes later; the final cardiac arrest rhythm was a slow, wide-QRS-complex PEA rhythm. Autopsy examination revealed acute, extensive inferoposterior MI with evidence of past large anterolateral MI.
Despite continued aggressive care of the patient in Case 2, the cardiac rhythm demonstrated further widening of the QRS complex with a slowed ventricular rate (Figure 4 [B]). No pulse was noted. Resuscitation was halted 10 minutes later; the final cardiac arrest rhythm was a slow, wide-QRS-complex PEA rhythm. Autopsy examination revealed acute, extensive inferoposterior MI with evidence of past large anterolateral MI.
1. Geddes LA, Roeder RA, Rundell AE, et al. The natural biochemical changes during ventricular fibrillation with cardiopulmonary resuscitation and the onset of postdefibrillation pulseless electrical activity. Am J Emerg Med. 2006;24:577-581.
2. Sutton-Tyrrell K, Abramson NS, Safar P, et al. Predictors of electromechanical dissociation during cardiac arrest. Ann Emerg Med. 1988;17:572-575.
3. Stueven HA, Aufderheide T, Waite EM, Mateer JR. Electromechanical dissociation: six years prehospital experience. Resuscitation. 1989;17:173-182.
4. Vanags B, Thakur RK, Stueven HA, et al. Interventions in the therapy of electromechanical dissociation. Resuscitation. 1989;17:163-171.
5. Edgren E, Kelsey S, Sutton K, Safar P. The presenting ECG pattern in survivors of cardiac arrest and its relation to the subsequent long-term survival. Brain Resuscitation Clinical Trial I Study Group. Acta Anesthesiol Scand. 1989; 33:265-271
6. Nadkarni VM, Larkin GL, Peberdy MA, et al. A decade of in-hospital resuscitation: outcomes and prediction of survival? Resuscitation. 2006;68:231-237.
7. Aufderheide TP, Thakur RK, Steuven HA, et al. Electrocardiographic characteristics in EMD. Resuscitation. 1989;17:183-193.
8. Stueven HA, Aufderheide T, Thakur RK, et al. Defining electromechanical dissociation: morphologic presentation. Resuscitation. 1989;17:195-203.


