Traumatic Brain Injury in the Elderly
This article is the fourth in a continuing series on trauma care and the older adult. The series discusses the growing problem of trauma in the elderly, including its causes and possible ways to prevent it, care in the acute stages, and manifestations and treatment strategies when trauma involves the torso, spine, brain, and hip. Authors include skilled experts in the trauma field representing various specialties at the R Adams Cowley Shock Trauma Center at the University of Maryland Medical Center and the University of Maryland School of Medicine.
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and disability in the United States. In the elderly, defined as persons age 65 years and older, trauma is the seventh leading cause of death, and TBI is responsible for over 80,000 Emergency Department (ED) visits annually.1 As the baby boom generation ages, the number of people over the age of 65 will reach 71 million by 2030 and represent 20% of the U.S. population. Between 2005 and 2050, the elderly population is projected to more than double. The population of those over the age of 85 years is expected to increase in a similar fashion to greater than 20 million in the same time period.2 As a result of this increase in the number of elderly persons, age-related TBI is expected to increase as well. In 2006, over $2.8 billion was spent on treating TBI in those older than age 65 years.3 Older age has been well recognized as an independent predictor of worse outcome after TBI, even with relatively minor head injuries.4,5
Epidemiology
Unintentional death was the ninth leading cause of death among those over age 65 years in 2006.6 A significant number of those deaths were from TBI. The leading causes of TBI in the elderly are falls (28%), motor vehicle crashes (20%), and struck by/against events, including pedestrians struck (19%).1 Most falls are from ground level and occur in or around the home.7 The rate of falls requiring hospitalization increases from 339 per 100,000 in people age 65-69 years old to 3637 per 100,000 in those age 85 years and older.8
Physiology of Traumatic Brain Injury
Trauma to the head results in mechanical damage to the parenchyma of the brain. Concussions, even without radiographic evidence of parenchymal damage, can lead to cerebral edema, increased intracranial pressure (ICP), and secondary ischemia.9 The initial insult occurs when force is transmitted to the brain tissue, which leads to cellular damage. The ensuing cascade of cellular changes leads to the cerebral edema and the secondary injury, which occurs over hours to days after the traumatic event. In addition to the direct parenchymal damage, the mechanical forces can lead to hemorrhage.
The intracranial space is a fixed volume. Once there is no more space to accommodate the extra volume of the mass, blood, or fluid, increased ICP will ensue. Various intrinsic mechanisms exist to prevent increases in ICP after trauma. These include shunting of the cerebrospinal fluid (CSF) to the spinal subarachnoid space, increasing CSF absorption, and shunting venous blood out of the skull.10 If this compensation fails, the ICP rises. Elevated ICPs are a marker for poor outcome. A mean ICP greater than 20 mm Hg has been shown to increase mortality.11,12
Cerebral blood flow is difficult to measure directly. Therefore, clinically, we follow the cerebral perfusion pressure (CPP). This is calculated as the difference between the mean arterial pressure (MAP) and the ICP (CPP = MAP-ICP). Normally, ICP ranges from 0 to 10 mm Hg. In people without TBI, autoregulation keeps cerebral blood flow constant, provided the person has a MAP between 50 and 150 mm Hg. Patients with TBI lose that mechanism of autoregulation, and thus the CPP is approximated by the MAP. As cerebral blood flow increases, the ICP also increases. Regarding TBI specifically, there may be decreased ability to autoregulate cerebral blood flow in elderly patients.4 It is imperative to prevent secondary insult in patients with TBI. Two sources of secondary insult, hypoxia and hypotension, have been demonstrated to be significantly associated with increases in morbidity and mortality after TBI.13
Descriptions of Traumatic Brain Injury
Focal contusions appear as small intraparenchymal punctate lesions initially but may lead to significant intracranial hypertension within a few days. Subdural hematomas (SDHs) result from rupture of cortical veins or arteries within the subdural space. In the course of aging, the dura becomes more adherent to the bone, and cerebral atrophy occurs. This leads to stretching of the bridging veins from the dura, predisposing them to bleeding. Epidural hematoma usually arises from a torn middle meningeal artery and is visible as blood between the dura and the skull. Skull fractures or bleeding venous sinuses can also lead to epidural hematomas.9 Additionally, the elderly seem more disposed to delayed presentation of acute SDH after trauma.14
Intracerebral hemorrhages or contusions are characterized by bleeding into the parenchyma of the brain. This can result in mass-like pressure on the surrounding parenchyma. Other hemorrhages that do not present as mass lesions include subarachnoid hemorrhage and intraventricular hemorrhages. In a subarachnoid hemorrhage, the blood spreads diffusely, thus not causing any mass effect. Intraventricular hemorrhage is a marker for the severity of trauma.15
Diffuse axonal injury (DAI) is a result of shearing of the axons in the white matter and brain stem from angular acceleration, such as when a car crash victim’s head fully extends, then fully hyperextends in rapid succession. It leads to an immediate decreased level of consciousness, yet rarely shows evidence of injury on the initial computed tomography (CT) scan and often requires magnetic resonance imaging (MRI) for diagnosis. Additionally, intracranial hypertension after DAI is rare.9
TBI is classified as mild, moderate, or severe depending on the initial Glasgow Coma Scale (GCS; Table I) and clinical symptoms. The GCS is one of the most prevalent scales used to assign a value to the components of the neurological exam. It is easy to remember, reproducible by different care providers, and provides a means to track changes in the exam. The components include eye opening, motor function, and verbal function. The three scores are added to yield a GCS score of 3-15. The motor function portion of the exam is the most important component for evaluating prognosis and change in exam.
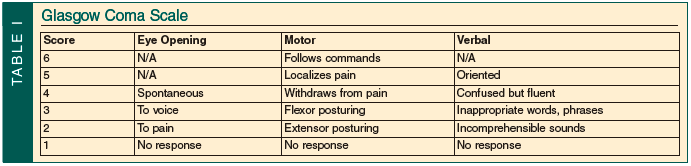
Mild TBI is the most common, accounting for 75% of cases. Patients with mild TBI may present with confusion or disorientation, perseveration, memory loss, and even loss of consciousness for less than 30 minutes.15 The GCS of these patients is 13-15. Moderate brain injury occurs with a GCS score of 9-12 and accounts for 10% of TBIs. Frequently, there will be abnormalities on CT scan. Surgical intervention is sometimes necessary. Stein and Ross16 demonstrated that 30% of patients admitted with a GCS of 9-13 had an intracranial lesion on CT, and 8% required neurosurgical intervention. They evaluated the group of patients with a GCS of 13 separately to demonstrate that these patients often have injuries and presentations more consistent with moderate head injury than mild head injury. Severe TBI is defined as a GCS of 8 or less. Mortality is much higher and functional recovery is much less likely in patients who suffer severe head injury, especially among the elderly.
Evaluation and Treatment
The initial management of patients with suspected TBI should start with an evaluation of their airway, breathing, and circulation. Patients with TBI who cannot protect their airway require endotracheal intubation. Once the airway is protected, the adequacy of the patient’s oxygenation and ventilation should be evaluated. Evaluation of circulation includes examination for external or internal sources of bleeding, as well as assessment of heart rate and blood pressure. Once hemodynamic stability and ongoing bleeding have been addressed, the clinician can evaluate the “disability” of the patient. This includes an initial assessment of level of consciousness. Assessing an elderly person can be difficult. Cognitive decline or dementia coupled with decreased hearing and visual acuity make the initial evaluation difficult. Often, family members can provide the best information regarding baseline confusion or dementia. The next most important measure of injury is whether or not the patient can follow simple commands. Other components of a neurological exam include evaluation of motor and sensory function bilaterally, pupil size, equality and response to light, eye opening, and for those in severe coma, the presence of a gag reflex, spontaneous respiratory effort, and corneal reflexes.
The third cranial nerve carries fibers that mediate pupillary constriction. As the brain herniates, these fibers can become subject to pressure that leads to a fixed and dilated pupil. Other causes of fixed and dilated pupils include midbrain injury and trauma directly to the eye or nerves. Pontine hemorrhages can produce pinpoint pupils.17
A CT scan of the brain is one of the most useful diagnostic tools available to evaluate patients with acute TBI. It can reveal hemorrhage that results in a mass lesion, cerebral edema, or herniation. Serial CT scans can be followed and, in conjunction with physical exam, help determine the need for surgical intervention. CT scans may provide the only evidence that TBI is serious, especially in elderly patients who can present with a high GCS score and significant hemorrhage.18 The atrophy of the brain associated with aging creates more space within the cranial vault for blood to accumulate before symptoms appear. Variations in practice exist among different institutions regarding the decision to obtain a CT scan in seemingly “normal acting” minor head trauma. The American College of Emergency Physicians recommends a head CT scan for any patient age 65 years or older who presents with mild head injury.19
A neurosurgical consult should be obtained when patients are diagnosed with TBI. During the initial observation period, the neurological exam should be repeated frequently. Clinical signs of increased ICP include bradycardia, headache, varying respirations, vomiting, and declining mental status. Changes in the pupil response or the components of the GCS can signal worsening brain injury. For instance, a reactive pupil that suddenly becomes dilated and unresponsive to light portends impending uncal herniation.15 If the patient’s GCS declines, especially on the motor or verbal portion of the exam, a stat head CT may show worsening mass effect from a hemorrhage. A worsening clinical exam should prompt an urgent discussion with the neurosurgical consultant.
Patients with moderate or severe TBI are managed in the Intensive Care Unit (ICU). As mentioned before, medical management of TBI is focused on preventing secondary insult. Blood pressure and oxygenation are monitored. Systolic blood pressure less than 90 mm Hg should be avoided, and PaO2 should be kept greater than 60 mm Hg. Other first-line measures to maintain an optimal ICP include keeping the head of bed elevated and appropriate sedation and pain control. Propofol is frequently administered for sedation. It has a quick onset and short duration of action, permitting frequent neurological examinations. Additionally, it may lower ICP. In patients with a contraindication to propofol, midazolam infusions can be administered.
If the ICP remains greater than 20 mm Hg in spite of these measures, hypertonic saline or mannitol can be administered.20 Mannitol is administered as a bolus of 0.25 to 1 mg/kg. Although the exact mechanism is still not known, it reduces ICP within a few minutes. It is an osmotic diuretic and should be used cautiously in patients with renal insufficiency. It is important to ensure that the patient does not become hypovolemic after the administration of mannitol. Hypertonic saline is effective in many concentrations. Three percent sodium chloride and 7.5% sodium acetate solutions are commonly used. The serum sodium and chloride concentrations are the limiting factors in the administration of hypertonic solution, as a metabolic acidosis can develop. If maximal medical therapy fails to control intracranial hypertension, barbiturate-induced coma or decompressive craniectomy are the next-line therapies.
In the past, barbiturates were frequently used for ICP control and sedation. Hypotension is a common side effect of barbiturate coma. Additionally, there are numerous drug interactions, as well as reports of increased rates of infection. A Cochrane review concluded that there is no evidence that barbiturate therapy improves outcome.21
Neurosurgical interventions may include placement of a device to measure ICP or brain tissue oxygenation. An intraventricular catheter (IVC) both measures ICP and can drain CSF. Draining CSF externally is a mechanical means of treating high ICP. Other neurosurgical interventions include evacuation of mass lesions, such as hematomas, with craniotomy or craniectomy. Cerebral edema that leads to intractable elevated ICP may be treated with decompressive craniectomy.22
Pre-Injury Anticoagulation and Antiplatelet Therapy
One of the more active areas of research in trauma is the effect of warfarin, clopidogrel, and aspirin on morbidity and mortality after TBI. There exists some controversy on the contribution of anticoagulation to the morbidity and mortality of elderly patients with TBI. In 2005, Gittleman et al23 found that seven of 89 patients with head trauma taking warfarin or heparin had intracranial hemorrhages (ICHs) on CT scan. Cohen and colleagues24 performed a prospective study to determine the severity of head injury, as well as the effect of anticoagulation, on outcome in anticoagulated geriatric patients. The authors found that patients with severe TBI had a mortality of 88%; patients with minor head injury (GCS score, 13-15) had an overall mortality of 80%. Interestingly, because of varying practice on the part of the treating physicians, not all of these patients received CT scans at presentation. The average international normalized ratio (INR) for this study was greater than 3, and the average age of the group was 77 years. Those two factors may contribute to the high mortality rate in this group of patients.
In 2003, Reynolds et al25 looked at 32 patients older than age 65 years taking warfarin with minor injuries above the clavicles and an initial GCS score of 15. There was wide variation in both CT scan findings and outcomes in these patients. At least two patients presented after discharge from the ED with significant ICH. The authors concluded that there is a 25% probability of ICH in elderly anticoagulated patients with a normal GCS score and minor trauma. Two studies that examined the effect of warfarin in elderly trauma patients showed no worse outcomes for those on anticoagulation.26,27 Neither of those studies isolated head injury from other trauma. Multiple subsequent studies demonstrate an increased risk of TBI and death from TBI in elderly patients on warfarin.28-30 The equivocal data on outcome in elderly trauma patients on warfarin prompted Pieracci and colleagues31 to look at the degree of anticoagulation. Overall mortality was 12% in this study, and patients with an ICH had a significantly higher mortality rate. Warfarin users were more likely to have a more severe head injury. The conclusions drawn in that study were that the use of warfarin leads to an increased injury severity, and the fact of being anticoagulated—not just being prescribed warfarin—leads to increased mortality.
Following the results of studies on anticoagulation, investigators began to study the effects of clopidogrel and aspirin. Mina et al30 found that patients on aspirin had an increased risk of death from TBI. Ivascu and colleagues32 found a high mortality rate among elderly persons who take aspirin, clopidogrel, or both. However, Fortuna et al33 found no increased mortality in elderly patients with TBI on aspirin and/or clopidogrel. However, age was found to be a significant predictor of mortality independent of antiplatelet and anticoagulant use.
Certainly, an INR should be obtained on any elderly person on anticoagulation therapy with a mechanism of injury that leads to TBI. Therapeutic and supratherapeutic INRs should be treated with fresh frozen plasma (FFP) and vitamin K or Factor VIIa in any suspected TBI. Ivascu et al34,35 demonstrated reduced mortality with a protocol for rapid reversal of anticoagulation. In their protocol, four units of FFP and 10 mg of intravenous vitamin K are administered immediately upon confirmation by head CT scan of ICH.34,35 Lankiewicz and colleagues36 demonstrated that using a prothrombin complex concentrate was also effective in rapidly reversing anticoagulation. Recombinant Factor VIIa has also been shown to decrease time to neurosurgical intervention and reduce the volume of FFP administered.37 The threshold for correction of INR remains variable among trauma surgeons.
There is less agreement on the administration of platelets in the face of ICH for patients on antiplatelet medications. Downey and colleagues38 showed no difference in mortality between patients who received platelets for ICH and those who did not.
Mortality, Rehabilitation, and Long-Term Disability
Elderly patients have a worse prognosis for similar injury severity than younger patients. As age increases, the prognosis worsens.39 However, some elderly patients with TBI will be discharged home from the hospital with good functional status, regardless of the injury severity. A 1992 study by Cagetti et al40 evaluated the outcome of 28 patients over age 80 years who had surgical intervention for either an acute SDH or epidural hematoma. The authors found that only three patients returned to pre-injury condition. Therefore, early, aggressive, evidence-based practice should dominate the early treatment of moderate and mild TBI. DeMaria and colleagues41 concluded that aggressive support of elderly trauma patients was justified as well. Survivors of blunt trauma over the age of 65 were studied after discharge. Thirty-three percent were living independently, while 30% required nursing home care. The nursing home cohort was older and had more severe injuries, including head and neck injuries, than the group that was discharged home.
Mosenthal et al39 evaluated the outcomes of adults with isolated head injury who were admitted to two level I trauma centers. Patients over age 65 had twice the in-hospital mortality of the young group. The mortality rate increased in the elderly group for each decade over age 50 years. Additionally, when patients were stratified according to severity of head injury, elderly patients had a higher mortality in all categories. Of survivors, elderly people were more likely to have severe disability or be in a persistent vegetative state. Additionally, the incidence of complications among the elderly was twice that of young patients during the hospitalization. There was no difference in length of stay or ICU days. The best independent predictors of mortality were age and admission GCS score.39
Susman et al5 looked at TBI in the elderly in New York State’s trauma database. The elderly patients had a lower injury severity score and, higher admissions mean GCS score as compared to nonelderly patients. Mortality in the elderly group was double that of the younger group, and mortality increased with increasing age, as in the Mosenthal et al39 study. Of the survivors, elderly patients had a lower GCS and increased disability, as measured by the Functional Independence Measure score at discharge.5
Elderly patients who present with a GCS score of less than 8 have an exceptionally poor prognosis. In a small retrospective review, Kilaru and colleagues42 examined a group of 40 elderly patients with TBI who presented with a GCS score of 8 or less. The total in-hospital survival was 13 of 40, representing 32%; yet, at 38-month follow-up, these patients had not shown neurologic improvement. Only three of the survivors lived functionally independent lives. In Australia, 428 elderly persons (> 65 yr) with isolated moderate-to-severe head injury were included in a study that examined predictors of outcome.43 Age, systolic blood pressure on arrival, and GCS score at arrival predicted outcomes. Only 16% of those with an initial GCS score of 3-8 survived. Approximately 52% of those with a GCS score of 9-12 survived hospitalization, and 65% of 310 survivors were followed up at six months. No patient with a GCS score of 3-8 was living independently at six months.43
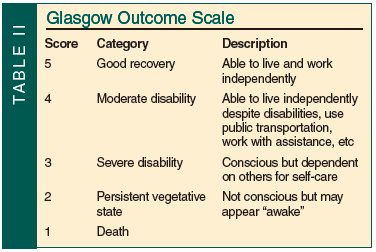 Elderly patients with mild TBIs who require more inpatient rehabilitation progress more slowly than younger patients. However, functional outcomes as measured by the Glasgow Outcome Scale (GOS; Table II) can be good. Sixty-eight percent of elderly patients with an initial GCS score of 13-15 achieved total independence.44 A less optimistic study found that patients over age 60 years who were discharged with a GOS score of 4 or lower had only a 37% chance of improving after one year of follow-up.45 Elderly patients incur longer hospital stays and are more likely to require acute rehabilitation than their younger counterparts. Even elderly patients with mild brain injury are more likely to suffer cognitive decline in the five years post-injury than younger patients.46
Elderly patients with mild TBIs who require more inpatient rehabilitation progress more slowly than younger patients. However, functional outcomes as measured by the Glasgow Outcome Scale (GOS; Table II) can be good. Sixty-eight percent of elderly patients with an initial GCS score of 13-15 achieved total independence.44 A less optimistic study found that patients over age 60 years who were discharged with a GOS score of 4 or lower had only a 37% chance of improving after one year of follow-up.45 Elderly patients incur longer hospital stays and are more likely to require acute rehabilitation than their younger counterparts. Even elderly patients with mild brain injury are more likely to suffer cognitive decline in the five years post-injury than younger patients.46
Conclusion
TBI is a major cause of morbidity and mortality among the elderly. As the population ages, physicians will need to know the appropriate evaluation and treatment of TBI in elderly patients. The use of anticoagulation and antiplatelet medication should be considered during the triage of elderly patients with mechanisms of injury concerning for TBI. Low-mechanism falls should warrant investigation of the possibility of head injury in elderly patients. The elderly have worse outcomes despite lower severity of injury; yet, aggressive, early, evidence-based management in a trauma center leads to best possible outcome. However, the best possible outcome is often rehabilitation or long-term skilled nursing, and not independent living.42
The authors report no relevant financial relationships.
Dr. Timmons is Surgical Critical Care Fellow, R Adams Cowley Shock Trauma Center, University of Maryland Medical Center; and Dr. Menaker is Assistant Professor, Department of Surgery, Division of Surgical/Critical Care, Program in Trauma, University of Maryland Medical Center, Medical Director, NeuroTrauma Intermediate Care Unit, and Physician Director of Quality Management, R Adams Cowley Shock Trauma Center. Dr. Gambert is Professor of Medicine and Co-Director, Division of Gerontology and Geriatric Medicine, Department of Medicine, University of Maryland School of Medicine, Director of Geriatric Medicine, University of Maryland Medical Center and R Adams Cowley Shock Trauma Center, and Professor of Medicine, Division of Gerontology and Geriatric Medicine, Johns Hopkins University School of Medicine, Baltimore, MD. Dr. Stein is Chief of Critical Care, R Adams Cowley Shock Trauma Center, and Associate Professor of Surgery, Department of Surgery, University of Maryland School of Medicine.
References
1. Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, Nation Center for Injury Prevention and Control; 2006.
2 .Current population reports. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. P25-1130. US Census Bureau. http://www.census.gov/prod/1/pop/p25-1130.pdf. Accessed March 15, 2010.
3. Statistics on hospital stays. Agency for Health Quality and Research. www.hcupnet.ahrq.gov. Accessed March 5, 2010.
4. Czosnyka M, Balestreri M, Steiner L, et al. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 2005;102(3):450-454.
5. Susman M, DiRusso SM, Sullivan T, et al. Traumatic brain injury in the elderly: Increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma 2002;53(2):219-224.
6. Deaths: Preliminary data for 2006. National Vital Statistics Report 2008;56:1-52.
7. Sjogren H, Bjornstig U. Injuries to the elderly in the traffic environment. Accid Anal Prev 1991;23(1):77-86.
8. Overall non-fatal injuries and rates per 100,000. 2007. United States. All races, both sexes, ages 65-85+ hospitalization. National Center of Injury Prevention and Control, Centers for Disease Control and Prevention. http://webappa.cdc.gov/sasweb/ncipc/nfirates2001.html. Accessed March 15, 2010.
9. Aarabi B, Mehta R, Eisenberg HM. Management of severe head injury. In: Moore AJ, Newell DW, eds. Neurosurgery: Principles and Practice. London: Springer-Verlag; 2005:369-379.
10. Vincent JL, Berre J. Primer on medical management of severe brain injury. Crit Care Med 2005;33(6):1392-1399.
11. Guillaume J, Janny P. Continuous intracranial manometry; physiopathologic and clinical significance of the method [article in undetermined language]. Presse Med 1951;59(45):953-955.
12. Balestreri M, Czosnya M, Hutchinson P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care 2006;4(1):8-13.
13. Rose J, Valtonen S, Jennett B. Avoidable factors contributing to death after head injury. Br Med J 1977;2(6087):615-618.
14. Itshayek E, Rosenthal G, Fraifeld S, et al. Delayed posttraumatic acute subdural hematoma in elderly patients on anticoagulation. Neurosurgery 2006;58(5):E851-E856.
15. Letarte P. The brain. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York: McGraw Hill Professional; 2008:397-418.
16. Stein SC, Ross SE. Moderate head injury: A guide to initial management. J Neurosurg 1992;77(4):562-564.
17. Sabates NR, Gonce MA, Farris BK. Neuro-opthamalogical findings in closed head trauma. J Clin Neuroopthalmol 1991;11(4):273-277.
18. Mandavia D, Newton K. Geriatric trauma. Emerg Med Clin North Am 1998;16(1):257-274.
19. Jagoda AS, Bazarian JJ, Bruns JJ Jr, et al; American College of Emergency Physicians; Centers for Disease Control and Prevention. Clinical policy: Neuroimaging and decision-making in adult mild traumatic brain injury in the acute setting. Ann Emerg Med 2008;52(6):714-748.
20. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and critical care for AANS/CNS, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotraum 2007;24(suppl 1):S55-S58.
21. Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Systematic Rev 1999;(3):CD000033.
22. Aarabi B, Hersdorffer DC, Ahn ES, et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg 2006;104(4):469-479.
23. Gittleman AM, Ortiz AO, Keating DP, Katz DS. Indications for CT in patients receiving anticoagulation after head trauma. AJNR Am J Neuroradiol 2005;26(3):603-606.
24. Cohen DB, Rinker C, Wilberger JE. Traumatic brain injury in anticoagulated patients. J Trauma 2006;60(3):553-557.
25. Reynolds FD, Dietz PA, Higgins D, Whitaker TS. Time to deterioration of the elderly, anticoagulated, minor head injury patient who presents without evidence of neurologic abnormality. J Trauma 2003;54(3):492-496.
26. Kennedy DM, Cipolle MD, Pasquale MD, et al. Impact of preinjury warfarin use in elderly trauma patients. J Trauma 2000;48(3):451-453.
27. Wojcik R, Cipolle MD, Seislove E, et al. Preinjury warfarin does not impact outcome in trauma patients. J Trauma 2001;51(6):1147-1152.
28. Howard JL 2nd, Cipolle MD, Horvat SA, et al. Pre-injury warfarin worsens outcome in elderly patients who fall from standing. J Trauma 2009;66(6):1518-1524.
29. Li J, Brown J, Levine M. Mild head injury, anticoagulants, and risk of intracranial injury. Lancet 2001;357(9258):771-772.
30. Mina AA, Knipfer JF, Park DY, et al. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma 2002;53(4):668-672.
31. Pieracci FM, Eachempati SR, Shou J, et al. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma 2007;63(3):525-530.
32. Ivascu FA, Howells GA, Junn FS, et al. Predictors of mortality in trauma patients with intracranial hemorrhage on pre-injury aspirin or clopidogrel. J Trauma 2008;65(4):785-788.
33. Fortuna GR, Mueller EW, James LE, et al. The impact of preinjury antiplatelet and anticoagulant pharmacotherapy on outcomes in elderly patients with hemorrhagic brain injury. Surgery 2008;144(4):598-603. Published Online: August 29, 2008.
34. Ivascu FA, Howells GA, Junn FS, et al. Rapid warfarin reversal in anticoagulated patients with traumatic intracranial hemorrhage reduces hemorrhage progression and mortality. J Trauma 2005;59(5):1131-1139.
35. Ivascu FA, Janczyk RJ, Junn FS, et al. Treatment of trauma patients with intracranial hemorrhage on preinjury warfarin. J Trauma 2006;61(2):318-321.
36. Lankiewicz MW, Hays J, Friedman KD, et al. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost 2006;4(5):967-970.
37. Stein DM, Dutton RP, Kramer ME, et al. Recombinant factor VIIa: Decreasing time to intervention in coagulopathic patients with severe traumatic brain injury. J Trauma 2008;64(3):620-628.
38. Downey DM, Monson B, Butler KL, et al. Does platelet administration affect mortality in elderly head-injured patients taking antiplatelet medications? Am Surgeon 2009;75(110):1100-1103.
39. Mosenthal AC, Lavery RF, Addis M, et al. Isolated traumatic brain injury: Age is an independent predictor of mortality and early outcome. J Trauma 2002;52(5):907-911.
40. Cagetti B, Cossu M, Pau A, et al. The outcome from acute subdural and epidural intracranial haematomas in very elderly patients. Br J Neurosurg 1992;6(3):227-231.
41. DeMaria EJ, Kenney PR, Merriam MA, et al. Aggressive trauma care benefits the elderly. J Trauma 1987;27(11):1200-120.
42. Kilaru S, Garb J, Emhoff T, et al. Long-term functional status and mortality of elderly patients with severe closed head injuries. J Trauma 1996;41(6):957-963.
43. Utomo WK, Gabbe BJ, Simpson PM, Cameron PA. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury 2009;40(9):973-977. Published Online: June 21, 2009.
44. Mosenthal AC, Livingston DH, Lavery RF, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma 2004;56(5):1042-1048.
45. Livingston DH, Lavery RF, Mosenthal AC, et al. Recovery at one year following isolated traumatic brain injury: A Western Trauma Association prospective multicenter trial. J Trauma 2005;59(6):1298-1304.
46. Marquez de la Plata CD, Hart T, Hammond FM, et al. Impact of age on long term recovery from traumatic brain injury. Arch Phys Med Rehab 2008;89(5):896-903.


