Transforming Your Primary Care Group Practice into a Center of Excellence in Type 2 Diabetes Care
Diabetes—a condition characterized by high levels of blood glucose caused by defects in insulin production, insulin action, or both—has become a nationwide epidemic, affecting an estimated 25.8 million Americans.1 Previously called non-insulin-dependent diabetes mellitus or adult-onset diabetes, type 2 diabetes accounts for 90% to 95% of diagnosed cases.1 In the United States, the burden of diabetes is enormous, largely due to related comorbidities and complications including cardiovascular disease (CVD), retinopathy, and kidney disease.1 Yet despite the availability of effective management strategies to mitigate the consequences of this disease, informational resources, and compelling evidence for intensive therapy, achievement of type 2 diabetes treatment goals remains alarmingly low.2
At the forefront of this epidemic are the primary care providers who treat approximately 90% of patients with type 2 diabetes.3,4 Empowering the primary care healthcare team with the knowledge and skills to combat type 2 diabetes and its related comorbidities is the most logical path to optimizing direct patient care. It is for these reasons that North American Center for Continuing Medical Education, LLC (NACCME) and Horizon CME—under the direction of Program Chairs Steven Edelman, MD, and Dace Trence, MD—sought to develop a high-impact educational program, rather than traditional, passive intervention, as an integral strategy for enabling primary care practitioners to overcome these and other barriers to optimal type 2 diabetes management.
The Transforming Your Primary Care Group Practice into a Center of Excellence in Type 2 Diabetes Care live, hands-on workshops were designed to provide the multidisciplinary team with an in-depth understanding of type 2 diabetes and practical approaches for combating the clinical inertia that often ensues with chronic disease management. Each 1- to 2-day workshop was hosted at a large-group primary care practice located in the “Diabetes Belt,” which includes Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia, and Mississippi.1 On day 1, a member of the faculty panel—each of whom is an expert in diabetes care—engaged the host physicians and their staff with an interactive discussion on the rationale and strategies for early and aggressive glucose management. Day 2 progressed with case-based instruction from the faculty expert to overcome provider and patient barriers to optimal therapy (offices that opted for a 1-day workshop began this portion of the program immediately after completing the interactive discussion with faculty described in day 1). The faculty mentor assisted the group in assessing current office practices with regard to diabetes management and areas that may be improved by implementing the knowledge and skills gained from the previous evening’s teachings. Throughout the workshop, the visiting faculty instructed participants through a mix of hands-on tutorials and interactive presentations, designed to provide a balance of expert-driven and learner-directed education.
As the series was limited to 15 large-group primary care practices in those states most affected by the diabetes epidemic, NACCME and Horizon CME have developed this monograph to broaden the activity’s reach to a nationwide audience and promote self-implementation of the program teachings to learners outside the live scope. This CE-accredited supplement includes an overview of the didactic, evidence-based presentation, as well as a practice-based case study outlining the events of a participating practice from evidence presentation, through initial assessment, and post-intervention conclusions. To further reinforce the goals of the program and the potential impact this initiative may have on patient outcomes, the case study also includes comments from a member of the faculty panel providing an interpretive assessment of the case example and instruction on self-implementation of best practices for overcoming common provider and patient barriers to optimal management of type 2 diabetes. It is our hope that readers gain insight into their own primary care practices and are able to put into action components of this series to transform their practices into centers of excellence in type 2 diabetes management.
Type 2 Diabetes: A Nationwide Epidemic
Type 2 diabetes affects a staggering number of individuals in the United States, resulting in a devastating clinical and economic burden. According to the Centers for Disease Control and Prevention (CDC), 8.3% of the US population has diabetes—an estimated 18.8 million individuals—while an additional 7 million Americans have diabetes but remain undiagnosed.1 The dramatic scope of this disease is emphasized by the number of patients diagnosed with diabetes in 1 year alone—approximately 1.9 million Americans received a diabetes diagnosis in 2010.5 In fact, the CDC estimates that 1 in 3 US adults will have diabetes by the year 2050.1 Clearly, immediate intervention is warranted to prevent the morbidity and mortality associated with this growing epidemic.
Clinical burden. The clinical impact of diabetes is exacerbated by concomitant disease-related microvascular and macrovascular complications such as CVD, retinopathy, nephropathy, and neuropathy.6 The rate of death due to heart disease, for example, is approximately 2 to 4 times higher for patients with diabetes compared with healthy adults.6 Similarly, the risk for stroke is 2 to 4 times higher among people with diabetes.6 In fact, in 2004, heart disease and stroke were noted on 68% and 16% of diabetes-related death certificates in people 65 years of age or older, respectively.6 Furthermore, diabetes is the leading cause of adult blindness, kidney failure, and nontraumatic lower-extremity amputations.6 Other clinical implications include biochemical imbalances that can cause acute life-threatening events, such as diabetic ketoacidosis; susceptibility to other illnesses; physical weakness or impairment; and an elevated risk of depression.6
Economic burden. Given this considerable clinical burden, it is not surprising that diabetes places a heavy financial strain on both individuals with the condition and the healthcare system at large. According to the CDC, the total cost of diabetes in 2007 was estimated at $174 billion, two-thirds of which was attributed to direct medical care.1 An analysis by the American Diabetes Association (ADA) reported that the average annual healthcare expenditures for individuals with diabetes totaled $13,243 compared with $2650 for those without diabetes, further emphasizing the dramatic effect of diabetes on medical costs.7 Notably, early and aggressive treatment of diabetes and existing diabetes-related complications may potentially limit further economic strain associated with this condition.8
Pathophysiology and Diagnosis
Understanding the pathogenesis of type 2 diabetes can help practitioners better recognize how to diagnose, manage, and treat this disease. The natural history of type 2 diabetes begins with impaired fasting glucose, a form of prediabetes in which blood glucose levels are 100 to 125 mg/dL following an overnight fast—higher than normal, but not yet high enough to be diagnosed as diabetes (ie, blood glucose measuring 126 mg/dL or higher following an overnight fast).9-11 As patients progress from impaired glucose tolerance (IGT) to type 2 diabetes, beta cells secrete additional insulin to compensate for the increasing insulin resistance.9-11 The physiologic response over time is a decrease in beta-cell function, so that at the point of diagnosis, patients with type 2 diabetes may have already lost at least 50% of the ability to secrete insulin.9,10,12 As the disease progresses, reduced functionality of beta cells continues, necessitating additional therapies, including insulin, to properly maintain glucose levels.12 In fact, one study of the pathogenesis of type 2 diabetes found that defects in insulin secretion and insulin action occur early in the disease process.13 Researchers identified a difference in insulin secretory responses between individuals who progressed to diabetes and those who maintained normal glucose tolerance (NGT) in the setting of increasing insulin resistance (ie, decreasing insulin sensitivity).13 In patients who maintained NGT, insulin secretion rose despite increasing insulin resistance; however, individuals who progressed to IGT and then to diabetes demonstrated a decline in insulin secretion and diminishing insulin sensitivity.13

Although determining beta-cell function in clinical practice may be difficult, patients at risk for, or suspected of, type 2 diabetes may be diagnosed via several measures (Figure 1).14-16 Clinicians often test hemoglobin A1c (HbA1c) levels as a screening tool for diabetes, followed by at least 2 fasting plasma glucose (FPG) evaluations to confirm the diagnosis.14 Although increased diabetes risk begins at an HbA1c of 5.7%, diabetes is diagnosed with an HbA1c of 6.5% or greater.14 Clinicians may also evaluate 2-hour plasma glucose using an oral glucose tolerance test, where IGT and diabetes are confirmed by results ranging between 140 mg/dL and 199 mg/dL and 200 mg/dL or greater, respectively.14 Although widely used in clinical practice, these tests have some notable limitations. For example, HbA1c may not be an accurate measure in African American, Asian, Hispanic, and other patients of non-European ancestry15; in addition, an elevated thyroid-stimulating hormone has been associated with falsely abnormal HbA1c.16 Furthermore, the oral glucose tolerance test may generate inconsistent results,17 suggesting that practitioners should consider multiple test results and hereditary and/or lifestyle factors when diagnosing diabetes. Yet, regardless of how the diagnosis of type 2 diabetes is confirmed, it is imperative to maintain proper glucose control over time and to escalate therapy as required to prevent diabetes-related complications.
Continued on next page
Getting Patients to Goal: A Focus on Glycemic Control
Glycemic control is critically important in the management of type 2 diabetes, given the microvascular and macrovascular complications associated with uncontrolled diabetes. This relationship was demonstrated by a prospective observational analysis of the United Kingdom Prospective Diabetes Study data, in which researchers found that a 1% decrease in HbA1c resulted in an average 21% reduction in diabetes-related death (P<.0001), a 37% reduction in microvascular complications (P<.0001), and a 14% reduction in myocardial infarction (P<.0001).11 Given the significant impact a reduction in HbA1c has on health outcomes, getting patients to their glycemic goals remains a pivotal focus in type 2 diabetes management.
Lifestyle interventions: diet and exercise. Lifestyle interventions comprise a key component in the achievement of glycemic control.18 The multicenter randomized Look AHEAD (Action for Health in Diabetes) study compared an intensive lifestyle intervention involving aggressive diet and exercise with group support featuring in-person and telephone follow-ups with diabetes support and education in 5145 overweight or obese patients with type 2 diabetes.19 Preliminary 4-year results have indicated that the intensive lifestyle intervention has prompted sustained weight loss and improvements in fitness, glycemic control, and CVD risk factors.19 Compared with the control group receiving only support and education, intervention participants have demonstrated a larger percentage decrease in HbA1c (-0.36% vs -0.09%, respectively; P<.001), as well as greater improvements in weight loss, treadmill fitness, systolic blood pressure, high-density lipo-protein cholesterol, and triglycerides (P<.001).19 Researchers are continuing to evaluate whether these risk factor differentials will generate differences in CVD-related events in patients with type 2 diabetes.19
Medical management. Although nonpharmacologic interventions are necessary and can be quite potent, the vast majority of patients with type 2 diabetes require pharmacotherapy to achieve glycemic control. Prior to initiating treatment, a basic understanding of the primary mechanisms of action of available therapies is useful. For instance, whereas biguanides work to decrease hepatic glucose production, sulfonylureas and short-acting insulin secretagogues increase insulin production in the pacreas.20-24 Thiazolidinediones provide glucose control by decreasing insulin resistance in muscle and adipose tissue and alpha-glucosidase inhibitors function by decreasing carbohydrate absorption in the instestine.20-24 Incretin mimetics provide glycemic control by imitating glucagon-like peptide-1 (GLP-1) and lower blood glucose by supplementing and/or replacing the activity of these endogenous incretins.20-24 Dipeptidyl peptidase-4 inhibitors—another incretin-based therapy—work to improve glucose control by indirectly increasing levels of intact, physiologically active endogenous GLP-1 and glucose-dependent insulinotropic polypeptide.20-24 Since patients often require escalation of therapy as their disease progresses, practitioners may improve patient outcomes by prescribing multiple agents with complementary mechanisms of action.
Treat-to-target therapy. Current best practices in diabetes care involve a treat-to-target approach in which medical therapy is initiated and then escalated with a view toward achieving specific HbA1c, preprandial plasma glucose, and peak postprandial plasma goals promulgated by the ADA and American Association of Clinical Endocrinologists (AACE) (Table).8,18,25,26 Although less stringent than the AACE recommendations, the ADA guidelines acknowledge that patients who are able to achieve a near-normal HbA1c level (<6%) without hypoglycemia may further reduce their risk of diabetes-related complications.26 Hypoglycemia is the primary limiting factor in glycemic management of diabetes; intensive treatment, therefore, is not appropriate for every patient including those who are elderly and all patients need to be aware of the increased risk of hypoglycemia.

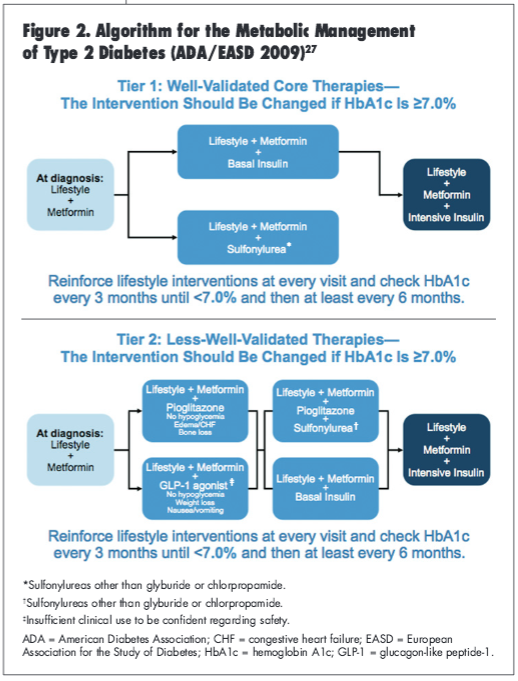
To assist practitioners in getting their patients to treatment goals, the ADA/European Association for the Study of Diabetes (EASD) consensus statement published in 2009 present an algorithm for the metabolic management of patients with type 2 diabetes (Figure 2).27 The guidelines provide a 2-tiered management approach.27 The well-validated approach (Tier 1) involves initiating therapy with metformin and lifestyle changes upon diagnosis, and the less—well-validated approach (Tier 2) offers several combination therapy options with the addition of therapeutic alternatives such as pioglitazone and GLP-1 agonists but also allows for the addition of intensive insulin therapy.27 With consideration of the suggested glucose control targets, the risk of hypoglycemia, and a variety of available pharmacotherapy, therapeutic recommendations should be carefully tailored for each individual patient and their glycemic goals.
Clinical Inertia and Other Barriers to Glycemic Control
Despite the existence of clinical practice guidelines that set forth specific targets for glycemic control, more than 60% of patients with type 2 diabetes remain uncontrolled.28 One causative factor is that therapies for managing blood glucose levels often fail over time.2,28-30 In addition, a major but perhaps underappreciated contributor to glycemic burden is that clinicians often wait too long to initiate pharmacotherapy and/or intensify treatment as it loses its effectiveness.2,28-30 This so-called clinical inertia reflects clinicians’ tendencies to keep patients on traditional oral agents (eg, metformin and sulfonylureas) as monotherapy or combination therapy for extended periods despite inadequate glycemic control rather than proactively intensifying therapy, initiating combination therapy earlier, and/or considering the use of newer agents with varying mechanisms of action.31
As treatment modifications are often not made proactively, but rather only when HbA1c reaches an unacceptably high level, prolonged exposure to hyperglycemia occurs; this reactive management approach carries meaningful clinical risk, given that even short periods of hyperglycemia increase the risk of complications.2 A prospective, population-based study using retrospective observational data quantified the amount of glycemic burden that accumulates when treatment fails.2 This study, conducted in members of the Kaiser Permanente Northwest Region, found that even in this generally well-controlled patient population, delays in treatment initiation and escalation resulted in an accumulation of excess glycemic burden of about 10 years with HbA1c greater than 7% and nearly 5 years with HbA1c higher than 8% from the point of diagnosis until starting insulin.2 The researchers concluded that clinicians should modify treatment regimens much more quickly and/or use treatments that are less likely to fail, including the use of insulin, where appropriate.2
Patients and providers often resist escalating to insulin therapy even when diabetes remains uncontrolled and a more aggressive approach is warranted. For example, a longitudinal observational cohort study conducted in 3891 patients with type 2 diabetes newly initiated on oral metformin/sulfonylurea combination therapy found that, over an average of 54.6 months, 41.9% of these patients added insulin and 11.8% received maximal doses of oral therapy.30 More than half of the oral therapy patients failed to maintain a target HbA1c level of 8%, yet continued on oral therapy for an average of nearly 3 years; this translated to a sustained glycemic burden of almost 32 months (HbA1c ≥9%).30 An additional 18% of patients never attained the 8% goal with oral therapy, but continued oral therapy for an average of 30 months, reaching a mean HbA1c level of 10%.30 Understanding why both patients and clinicians allow such delays in escalation of therapy is essential to overcoming treatment barriers and providing excellent diabetes care.
A number of other barriers constitute challenges to maintaining glycemic control.32-35 These barriers include, but are not limited to, the progressive nature of type 2 diabetes; ineffective diet and exercise initiatives; lack of or diminishing efficacy of pharmacologic agents; poor patient literacy, numeracy, and/or education; patient nonadherence to therapy; culture and language barriers; competing demands on physician time (ie, multiple patient comorbidities to be addressed during the visit); adverse events related to therapies; healthcare system factors; and cost of medical care.32-35 Patient-related barriers to insulin therapy often include the belief that the need to use insulin is a personal failure; the belief that insulin is not effective or that it causes complications or death; fear of hypoglycemia, weight gain, loss of independence, and/or injection pain; and overall cost.36 Clinicians can alleviate some of these obstacles by asking patients about their specific concerns, providing counseling and educational materials, and making referrals to clinical support staff members such as registered dietitians and certified diabetes educators who can provide additional education and facilitate problem-solving and coping skills.
Continued on next page
Initiating Insulin Therapy in Type 2 Diabetes
Management strategies for patients with type 2 diabetes attempt to replicate physiologic insulin secretion, and thus require a focus on both postprandial and basal requirements. The normal physiologic response of glucose and insulin to meals involves insulin release in response to nutrient ingestion; in contrast, basal insulin is continuously secreted over a 24-hour period.37 This underlying physiology underscores patients’ need for both basal and meal-time insulin. In the past, the pharmacokinetics of the various insulin formulations did not adequately duplicate these profiles; however, newer insulin analogs provide more appropriate physiologic profiles.38
Insulin initiation in type 2 diabetes is indicated in a variety of scenarios, such as with high blood glucose levels despite maximal glucose-lowering medication therapy; unintentional weight loss despite maximal therapy; very high blood glucose at diagnosis (glucose toxicity); at the start of pregnancy; and in hospitalized patients with diabetes.39 The use of insulin therapy offers several advantages, including the ability to overcome glucose toxicity in patients with high blood glucose levels, control fasting glucose and thus improve day-long glycemic control, and tailor therapy to individualized patient goals to maintain safety.39 Furthermore, using insulin treatment early in the natural history of the disease can optimize and/or replace first-phase insulin release; later in the disease course, treatment can replicate both basal and prandial insulin patterns depending upon a patient’s physiologic needs.39
Targeting both fasting and postprandial plasma glucose (PPG) is often required to optimize blood glucose control, especially in patients with uncontrolled diabetes. A study evaluating the relative contribution of fasting and PPG to hyperglycemia in 290 noninsulin-, nonacarbose—using patients with type 2 diabetes suggested that both contribute to overall hyperglycemia.40 Researchers found that the relative contribution of PPG levels decreased progressively from the lowest to the highest quintile of HbA1c (P<.001); in contrast, the relative contribution of FPG showed a gradual increase as HbA1c levels increased (P<.001).40 These findings may reveal which agents are more likely to be effective given a patient’s level of glycemic control: treatment of patients with near-controlled diabetes might focus on PPG, whereas treatment of both fasting and PPG levels is equally important in patients with higher HbA1c levels.40
Insulin options. A number of factors influence the selection of insulin and the strategy for initiating insulin therapy. The patient’s particular medical needs and treatment goals are a primary factor; the clinician should consider the patient’s HbA1c level and the distance from target, as well as the PPG level. For instance, the action profiles of injectable insulins vary in onset of action, peak, and duration such that insulin regimens will vary based on the needs of the patient.41 Flexibility in designing individualized treatment regimens is critical, given that a regimen that works well for one patient might be unsuccessful in another. In addition, escalation of therapy must be pursued safely, with a view toward avoiding hypoglycemia. Additional patient-specific issues to consider include medical literacy and judgment, psychosocial and cultural considerations, physical capabilities and limitations (eg, dexterity limitations, vision impairment), and other medical conditions and issues relating to the use of noninsulin medications.39
Rapid-acting insulins. Rapid-acting insulins—namely aspart, glulisine, and lispro—are created by making minor amino acid alterations to the human insulin molecule; their pharmacokinetic profiles closely mimic endogenous insulin, and they tend to have a relatively low risk of hypoglycemia.42 These small alterations reduce the tendency to aggregate into pairs or groups of molecules, thus speeding absorption and closely matching normal mealtime absorption patterns, particularly the timing of carbohydrate absorption.42,43 When administered 15 to 30 minutes prior to meals, clinical studies have shown that the quick onset of action matches normal mealtime peaks of plasma insulin better than regular human insulin (RHI), leading to less prominent PPG peaks and fewer incidences of late postprandial hypoglycemia.42,43 However, it is important to be aware that the rapid waning of the effects of mealtime rapid-acting insulin leads to greater dependency on adequate basal insulin levels between meals and overnight.42,43
Researchers have found that rapid-acting insulin analogs provide better control of HbA1c and PPG than RHI.38,39 A meta-analysis of 13 randomized controlled trials, for instance, found that rapid-acting analogs reduced HbA1c by 0.4% more compared with RHI in patients with type 2 diabetes (P=.027), with a similar rate of severe hypoglycemia.44 In a multicenter, randomized, open-label, parallel-group, 6-month study (n=882) with a 6-month extension period (n=714) that compared the long-term efficacy and safety of insulin aspart versus RHI in patients with type 1 diabetes for at least 18 months, PPG levels were significantly lower in patients receiving insulin aspart compared with those prescribed RHI (P<.05).45 Mean HbA1c values were also slightly lower in the insulin aspart group compared with the RHI cohort (7.78% vs 7.93%, respectively; P=.005).39,45
Basal insulins. With no distinct peak effect, basal insulins such as glargine and detemir mimic the natural pancreatic basal insulin secretory pattern by providing a continuous effect up to 24 hours.39,41 Neutral protamine Hagedorn (NPH), another basal insulin option, also mimics natural insulin patterns, although its effect is slightly shorter at 10 to 16 hours.39 Basal insulins have several desirable characteristics, including that these agents minimize the risk of nocturnal hypoglycemia and provide a reliable absorption pattern largely due to improved patient adherence facilitated by once- or twice-daily administration (ie, glargine and detemir, respectively).46,47
Available data indicate that basal insulins are relatively efficacious, although some differences do exist between agents.46,47 A 24-week treat-to-target trial, for example, randomized insulin-naive patients with type 2 diabetes to glargine once daily or detemir twice daily (n=973).46 Reporting HbA1c improvements of -1.46% and -1.54% for glargine and detemir, respectively, the agents were found to be equally effective in achieving glycemic control (P=.149).46 Similar percentages of patients achieved an HbA1c less than 7% (P=.254)—although a greater percentage of patients administered detemir reached an HbA1c less than 6.5% (P=.017)—and the risk of hypoglycemia was also comparable.46
Insulin glargine in combination with oral therapy has also been shown to improve HbA1c without prompting hypoglycemia.47 In a randomized, open-label, parallel-group, 24-week multicenter trial, 756 overweight men and women with inadequate glycemic control (HbA1c >7.5%) on 1 or 2 oral agents were randomized to receive glargine or NPH once daily.47 The researchers reported that HbA1c levels were similar with glargine and NPH (6.96% vs 6.97%, respectively), and approximately 60% of patients attained an HbA1c of 7% or less with each agent.47 However, glargine was associated with significantly less hypoglycemia, with documented nocturnal hypoglycemia found to be nearly 25% higher with NPH compared with glargine (33.2% vs 26.7%, respectively; P<.05).47
Split-mixed insulin therapy. Recent data suggest that glycemic variability contributes to the risk of microvascular complications; therefore, focusing solely on basal insulin may not be an ideal medical management strategy.48 Instead, nearly all patients with severe insulin deficiency will require insulin replacement with both basal and prandial therapies.48 Split-mixed insulin therapy—a combination of a rapid- and intermediate-acting or regular insulin such as 50% NPH/50% regular insulin and 70% NPH/30% regular insulin—is relatively easy to use and covers insulin requirements throughout most of the day.48,49 As split-mixed insulin therapy does not offer an accurate replication of physiologic patterns, it is associated with a greater likelihood of nocturnal hypoglycemia from the peak of presupper NPH, as well as an increased chance of fasting hyperglycemia as the effect of pre-supper NPH wanes.48,49
For patients who have difficulty mixing insulin within a syringe, premixed insulin analogs may be a preferable option. Premixed insulins, such as biphasic insulin aspart 70/30 and insulin lispro 25/75, provide rapid- and intermediate-acting insulin in a single injection administered via a pen device. Premixed insulin offers the ability to intensify therapy from a starting regimen of 1 injection of premixed insulin to 2 or 3 injections of the same insulin; however, use of premixed insulin requires a relatively consistent meal and exercise pattern, as the ratio of rapid- to intermediate-acting insulin is fixed.50,51
Research has shown that use of a twice-daily premixed insulin can be more effective in achieving HbA1c targets than once-daily glargine.50 A 28-week parallel-group study randomized insulin-naive patients with an HbA1c of 8% or more on metformin alone or in combination with other oral therapies to either biphasic insulin aspart or insulin glargine (n=233); the results demonstrated that HbA1c was lower in the biphasic insulin aspart group compared with the glargine cohort (6.91% vs 7.41%, respectively; P<.01), and that the decline in HbA1c was greater in the biphasic insulin aspart group (-2.79% vs -2.36%, respectively; P<.01).50 Furthermore, significantly more patients receiving biphasic insulin aspart reached target HbA1c values compared with those treated with glargine (P<.001).50 Notably, hypoglycemia (P<.05) and weight gain (P<.01) were significantly greater with the premixed formulation compared with basal insulin.50 As such, although better control of glucose is expected with premixed insulin with regard to PPG and FPG, it is generally not preferred due to the higher risk for hypoglycemia and weight gain.
Basal-bolus treatment. Indications for basal-bolus treatment include significant insulinopenia; glucose pattern instability (usually the result of significant insulinopenia); hypoglycemia; lifestyle needs (ie, variable schedules that necessitate the need for a simplified insulin program); an inability to achieve therapeutic goals requiring an intensive insulin regimen; and the need for weight loss.39 Appropriate dosing depends on the patient’s sensitivity to insulin and often his or her carbohydrate load, as carbohydrates are the food component that most affect blood glucose.39 Patients utilizing a basal-bolus approach should be instructed to count carbohydrates by tracking the grams of carbohydrates consumed for the purpose of adjusting insulin dosing—understanding that the more carbohydrates consumed, the more insulin they will require—and to monitor both pre- and PPG.39
Despite requiring more work on the part of the patient to determine the appropriate dosage, the basal-bolus approach can effectively reduce HbA1c in patients with type 2 diabetes.52 A multicenter, open-label, noninferiority trial randomized patients with type 2 diabetes who were previously treated with insulin glargine plus oral agents to either prandial premixed lispro or basal-bolus therapy (n=374).52 After 24 weeks, basal-bolus therapy was associated with a lower HbA1c (6.78% vs 6.95%, respectively; P=.021), although HbA1c declined significantly from baseline with both therapies (P<.0001) with similar rates of hypoglycemia.52
Typically, the basal-bolus approach involves 3 preprandial injections; however, recent evidence suggests that a stepwise approach may be an effective alternative.53 In an open-label, parallel-group study, 343 adults with type 2 diabetes on oral antidiabetic therapy were randomized to a 14-week run-in with insulin glargine and then randomly assigned to receive insulin glulisine either 1, 2, or 3 times daily for 24 weeks.53 During the randomization phase, HbA1c reductions with insulin glulisine once or twice daily were similar to those achieved with insulin glulisine 3 times daily (P>.5), although more patients met target HbA1c levels with 3 injections (46%) compared with 2 injections (33%) or 1 injection (30%).53 Severe hypoglycemia occurred in more patients receiving 3 preprandial injections than those receiving 2 or 1 injection (16%, 8%, and 7%, respectively), but these differences were not significant.53
Initiating and advancing insulin treatment. Insulin should be initiated at a low dose to determine insulin sensitivity and avoid inducing hypoglycemia.47 The ADA/EASD consensus algorithm for the initiation and adjustment of basal insulin suggests starting with an intermediate-acting insulin at bedtime or a long-acting insulin at bedtime or in the morning at a dose of 10 units or 0.2 units per kilogram.27 Patients should be advised to check their glucose daily and increase the dose by 2 units every 3 days until levels are consistently in the target range (ie, FPG 70-130 mg/dL); increasing the dose by larger increments (up to 4 units every 3 days) is permissible if fasting glucose is greater than 180 mg/dL.27 Some practitioners and patients may prefer an even simpler titration option, where the dose is increased by 1 unit every day until FPG is at most 120 mg/dL.54 When using this approach, the dosage should not be increased for 1 week if there are any episodes of documented hypoglycemia (<72 mg/dL) during the preceding week.54 Regardless of the titration method used, the glycemic goal must be individualized to the patient to avoid hypoglycemia.
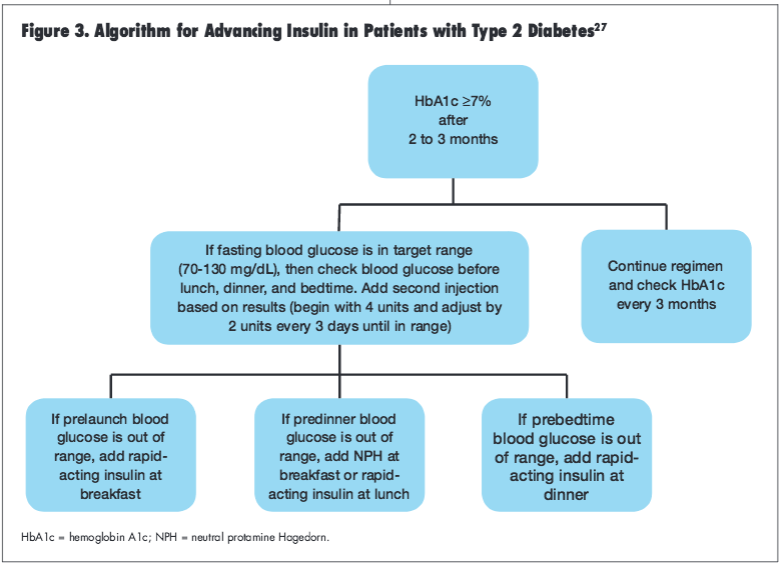
If a patient’s HbA1c is 7% or higher 2 to 3 months after initiating insulin therapy, the ADA/EASD consensus statement suggest advancing the treatment regimen by adding a rapid-acting insulin prior to breakfast, lunch, or dinner, depending upon blood glucose levels (Figure 3).27 Notably, once a patient advances to more than 1 insulin injection daily, use of insulin secretagogues may be discontinued (particularly when using a rapid-acting preprandial insulin) although continuation of metformin therapy is permissible.55,56
Continued on next page
Strategies for Reducing Barriers to Insulin Therapy
The transition from oral to insulin therapy for the management of type 2 diabetes is often met with resistance on the part of the patient, a situation that can often be easily rectified with basic patient- and provider-centered strategies.36 Foremost among these techniques is patient education and counseling; clinicians need to explain the progressive nature of type 2 diabetes and beta-cell failure to prevent and/or diminish the erroneous belief that insulin use is a personal failure, and point out that almost
everyone with type 2 diabetes eventually requires insulin therapy.39 Relaying the benefits associated with newer formulations may also help to overcome some of these barriers, as newer formulations have been shown to cause less hypoglycemia, weight gain, and injection pain and may
actually improve quality of life.57
Involvement of the entire healthcare team is particularly helpful for successfully starting an insulin regimen. Physicians can refer patients to a dietitian and certified diabetes educator, who can help them identify strategies to prevent weight gain and provide self-management education strategies, respectively. Staff nurses can assist by addressing concerns about injection pain by noting that current needles are very small and that many patients find insulin administration to be less painful than blood glucose testing; they can also teach proper injection technique to minimize discomfort. Other specific strategies to facilitate the successful implementation of insulin therapy include carbohydrate counting, use of insulin pen devices, and ongoing glucose monitoring.
Carbohydrate counting. With the help of a registered dietitian, individuals with type 2 diabetes can learn how to count carbohydrates. Carbohydrate counting is particularly useful for people who have considerable variability in their carbohydrate intake and who use variable preprandial doses of rapid-acting insulin, since the insulin dose must be adjusted upward if foods with a high carbohydrate content are consumed. Pre- and PPG checks can be useful to indicate the number of insulin units required for a given number of carbohydrate grams ingested.58
Use of insulin pens. The administration of insulin has become more user-friendly with the development of a variety of prefilled, disposable “pen” insulin delivery devices. These pens are easier and more appealing to use than syringes but offer similar safety and efficacy; patients have reported that pen devices are less painful than traditional needles and allow for more discrete administration, thereby minimizing any perceived social stigma regarding administering insulin in the presence of others.53 In addition, patients using pen devices benefit from improved confidence in dose-setting, largely due to the increased readability of pen scales.59
Glucose monitoring. Glucose monitoring is essential in type 2 diabetes management.60 Thus, education is required to teach patients how to interpret glucose test results and respond accordingly. Record-keeping is of critical importance to the monitoring process; glucometers now have memory devices that facilitate glucose level tracking, and “smart phone” applications are being developed to facilitate data tracking and retrieval. Practitioners should discuss the best method for tracking these numbers, again making certain that all components of the treatment plan are individualized to the patient.60
Conclusion
Type 2 diabetes is a chronic condition, yet its implications can be mitigated through proactive escalation of therapy, including early advancement to combination therapy and/or insulin use. Avoiding clinical inertia in diabetes management—particularly in the primary care setting where patients with type 2 diabetes are most likely to seek treatment—by aggressively assessing the need for therapeutic escalation can help physicians and the entire healthcare team improve clinical outcomes for patients with diabetes.
References
1. Centers for Disease Control and Prevention. Chronic disease prevention and health promotion. Diabetes successes and opportunities for population-based prevention and control, at a glance 2011. www.cdc.gov/chronicdisease/resources/publications/AAG/ddt.htm. Accessed February 21, 2012.
2. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
3. Vinik A. Advancing therapy in type 2 diabetes mellitus with early, comprehensive progression from oral agents to insulin therapy. Clin Ther. 2007;29:1236-1253.
4. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):
2545-2559.
5. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics Fact Sheet. United States Department of Health and Human Services, National Institutes of Health; 2011.
6. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). National diabetes statistics, 2011. http://diabetes.niddk.nih.gov/dm/pubs/statistics/#Kidney. Accessed February 21, 2012.
7. Hogan P, Dall T, Nikolov P; American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26(3):917-932.
8. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187-192.
9. Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care. 1999;26(4):771-789.
10. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347(17):1342-1349.
11. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35):
prospective observational study. BMJ. 2000;321(7258):405-412.
12. Lebovitz H. Type 2 diabetes: an overview. Clin Chem. 1999;45(8):1339-1345.
13. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787-794.
14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62-S69.
15. Dagogo-Jack S. Pitfalls in the use of HbA1(c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol. 2010;6(10):589-593.
16. Kim MK, Kwon HS, Baek KH, et al. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010;33(12):2546-2548.
17. Chalew SA, Koetter H, Hoffman S, Levin PA, Kowarski AA. Diagnosis of reactive hypoglycemia: pitfalls in the use of the oral glucose tolerance test. South Med J. 1986;79(3):285-287.
18. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11-S61.
19. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566-1575.
20. Fonseca V. The CADRE Handbook of Diabetes Management. New York, NY: Medical Information Press; 2004.
21. Lacy CF, et al. Drug Information Handbook, 20th ed. Hudson, OH: Lexi-Comp, Inc; 2011.
22. Mest HJ, Mentlein R. Dipeptidyl peptidase inhibitors as new drugs for the treatment of type 2 diabetes. Diabetologia. 2005;48(4):616-620.
23. Bray GM. Exenatide. Am J Health Syst Pharm. 2006;63(5):411-418.
24. Pijl H, Ohashi S, Matsuda M, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23(8):1154-1161.
25. Rodbard HW, Blonde L, Braithwaite SS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):3-68.
26. American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24(4):775-778.
27. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
28. Del Prato S, Felton AM, Munro N, et al. Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract. 2005;59(11):1345-1355.
29. Campbell IW. Need for intensive early glycaemic control in patients with type 2 diabetes. Br J Cardiol. 2000;7:625-631.
30. Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen
Intern Med. 2007;22(4):453-458.
31. Vigersky RA. An overview of management issues in adult patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(2):245-250.
32. Del Prato S, Penno G, Miccoli R. Changing the treatment paradigm for type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S217-S222.
33. Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30(4):807-812.
34. Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196-201.
35. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-1224.
36. Funnell MM. Overcoming barriers to the initiation of insulin therapy. Clin Diabetes. 2007;25(1):36-38.
37. McCall AL. Insulin therapy and hypoglycemia. In: Leahy JL, Cefalu WT, eds. New York, NY: Marcel Dekker, Inc; 2002:193-222.
38. Freeman JS. Are analogue insulins superior to human insulin in clinical practice? Curr Diab Rep. 2010;10(3):176-183.
39. Mayfield JA, White RD. Insulin therapy for type 2 diabetes: rescue, augmentation, and replacement of beta-cell function. Am Fam Physician. 2004;70:
489-500,511-512.
40. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2
diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
41. Flood TM. Appropriate use of insulin analogs in an increasingly complex type 2 diabetes mellitus (T2DM) landscape. J Fam Pract. 2007;56(1 Suppl):
S1-S10.
42. Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart,
lispro, and glulisine. Endocr Pract. 2011;17(2):271-280.
43. Sheldon B, Russell-Jones D, Wright J. Insulin analogues: an example of applied medical science. Diabetes Obes Metab. 2009;11(1):5-19.
44. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs. regular human insulin in type 2 diabetes: a meta-analysis. Diabetes Obes Metab. 2009;11(1):53-59.
45. Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care. 2000;23(5):583-588.
46. Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin
detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33(6):1176-1178.
47. Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH
insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-3086.
48. Hirsch IB. Intensifying insulin therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(Suppl 5A):21S-26S.
49. Leroith D. Our evolving understanding of getting to goal using insulin in type 2 diabetes. Endocrinol Metab Clin North Am. 2007;36(Suppl 2):9-19.
50. Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260-265.
51. Malone JK, Bai S, Campaigne MN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med. 2005;22(4):374-381.
52. Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31(1):20-25.
53. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17(3):395-403.
54. Gerstein HC, Gerstein HC, Yale JF, et al. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabetes Med. 2006;23(7):736-742.
55. Hirsch IB, Yuan H, Campaigne BN, Tan MH. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract. 2009;15(4):343-348.
56. Riddle MC. Starting and advancing insulin for type 2 diabetes: algorithms and individualized methods are both necessary. J Clin Endocrinol Metab. 2008;93(2):372-374.
57. Heinemann L. Overcoming obstacles: new management options. Eur J Endocrinol. 2004;151(Suppl 2):T23-T27.
58. Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care. 2008;31(7):1305-1310.
59. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18-S24.
60. American Diabetes Association. Diabetes Care. 2012;35(Suppl 1):S11-S63.


