Pulmonary Rehabilitation: To Refer or Not to Refer?
Chronic obstructive pulmonary disease (COPD) is the third-leading cause of death in the United States and the second-leading cause of disability.1 Substantial social and economic costs are associated with COPD, costs that are expected to increase as the morbidity and mortality rates continue to rise in the coming decades.2 In 2010, COPD costs in the United States totaled roughly $50 billion, which includes $29.5 billion in direct healthcare costs.3 By comparison, breast cancer and lung cancer are responsible for $16.5 billion and $12.1 billion in direct healthcare costs, respectively.4 By the year 2020, direct medical costs of COPD are projected to reach approximately $49 billion annually.5
The (Underutilized) Solution
Pulmonary rehabilitation (PR) can transform the lives of patients who suffer from COPD, and is an essential component of comprehensive COPD care. However, despite evidence that PR improves exercise capacity and health-related quality of life, as well as reduces dyspnea, anxiety, depression, and healthcare utilization—and is part of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for managing a patient with COPD—referrals to pulmonary rehabilitation programs remain dismally low. Primary care providers must recognize their role as advocates and educators for PR.
__________________________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Healthy Diet Could Decrease COPD Risk
Emphysema Could Increase Risk of Death In Certain Groups
__________________________________________________________________________________________________________________________________________________________________
The Case for Pulmonary Rehabilitation
PR is an encompassing, nonpharmacological intervention for patients with chronic lung disease. It is defined by the American Thoracic Society and the European Respiratory Society as “a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, and behavior change, designed to reduce dyspnea, improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors.”6
PR has many proven and sustainable economic benefits for patients. By decreasing healthcare utilization, including hospital admissions, overall expenses are reduced for the patient. A significant decline in both COPD exacerbation and hospitalization frequency was found after participation in PR, with the mean number of acute exacerbations of COPD showing a decrease of 1.37 events annually. Additionally, the number of hospitalizations decreased by 0.68 visits.7
COPD has dominated PR research and published experience. However, holding onto this viewpoint can be a pitfall in caring for patients with chronic lung diseases. PR is effective treatment for the downward spiral of dyspnea and inactivity from various other chronic lung diseases, including asthma, interstitial lung disease, and pulmonary arterial hypertension—before and after lung transplantation or lung volume reduction surgery.6 Despite published evidence of PR efficacy in COPD and perennial recommendation by worldwide guidelines, referrals remain alarmingly low (Figure 1).

The Plight of the Patient with Chronic Lung Disease
Patients with COPD and other chronic lung diseases experience a downward spiral of dyspnea (shortness of breath) or dyspneic (shortness of breath from a level of exercise, such as walking on level ground, that would not ordinarily cause the reaction in healthy individuals). The patient’s skeletal muscles become tense, making it harder to take a deep breath, and they become worried and possibly panic, which further aggravates their condition and anxiety.8
To avoid this cycle, and public embarrassment, patients with chronic lung disease tend to limit activities that cause dyspnea, often staying at home in isolation.9 The limits on activity that result from dyspnea can lead to a sedentary lifestyle, which can foster progressive social detachment and isolation, physical deconditioning, eventual loss of skeletal muscle mass, loss of cardiovascular function, and inevitably even greater dyspnea.10 In addition, a loss of activity in COPD patients is associated with increased morbidity and mortality.11,12
(The Role of the Practitioner and How To Avoid Pitfalls on next page)The Role of the Practitioner
The initial evaluation for PR is actually a consultation that begins with a complete assessment of the patient’s symptoms and risks from COPD, including acute exacerbations, review of medical history including comorbidities (ie, gastroesophageal reflux disease and obstructive sleep apnea), physical examination, and exercise evaluation. Patients are given standardized questionnaires that collect vital information regarding symptoms, activities, emotional and social well-being, and health-related quality of life measures at both the initiation and conclusion of the program in order to ascertain changes that may occur as a result of the program.
The infrastructure of a PR program includes, but is not limited to, individualized and supervised exercise training for lower and upper body, education, symptom monitoring, behavioral change, psychosocial support, nutrition, advanced care planning, and care coordination. Patient education includes teaching lung anatomy and pathophysiology, breathing retraining, recognition of lung infection and their prevention with action plans for worsening symptoms and acute exacerbations, nutrition, medications and their adverse effects, benefits of daily exercise, conservation of energy, smoking cessation, and avoidance of known triggers of shortness of breath.
An individualized treatment plan is designed to address the specific needs of the patient for each component. The components of a PR program are all-inclusive, increase the complexity of care, and warrant collaboration by providers in multiple disciplines dedicated to reducing symptoms and risks from COPD, including physicians, respiratory therapists, nurses, physical therapists, exercise therapists, dieticians, psychologists, social workers, and pharmacists.6 This collaboration is necessary to meet the needs of patients and their families. The University of California Davis Health System has expanded PR by integrating primary palliative care into their comprehensive program (Figure 2).

A personalized exercise prescription is generated from the exercise evaluation and other functional tests, such as the shuttle walk test or the 6-minute walk test, to safely guide the exercise progression of the patient. The regimen typically comprises exercise endurance training and strength exercises, but can also include interval and resistance training as well as balance and meditation exercises.8 PR can improve exercise capacity, strength, and endurance of the upper limbs, and improves arm function. It also increases peak workload, peak oxygen consumption, and endurance time.13 Research also has shown that respiratory muscle training when combined with exercise training can be beneficial.2
Implementing a proper exercise regimen can give patients the means to a more active lifestyle by reducing dyspnea, versus a sedentary lifestyle. Overall, PR has been shown to significantly reduce perceived dyspnea.2
To minimize the negative impact of dyspnea, patients are taught how to manage their symptoms and subsequent anxiety. Using breathing retraining techniques that include pursed-lip breathing and diaphragmatic breathing, patients can often alleviate their symptoms. Ensuring that patients understand the best practice rescue positions for relief of dyspnea and fatigue empowers them to control breathing while at rest or following exertion.
Patients are also taught the importance of energy conservation. This is achieved through limiting their amount of work, planning ahead, organizing their environment, positioning their bodies for comfort and efficiency, taking control of time, using tools that make work easier, and tending to their psychological hygiene.14 These types of self-management strategies support self-efficacy and enable patients to have better control over their chronic lung disease and comorbidities.6
Barriers to Patient Referrals
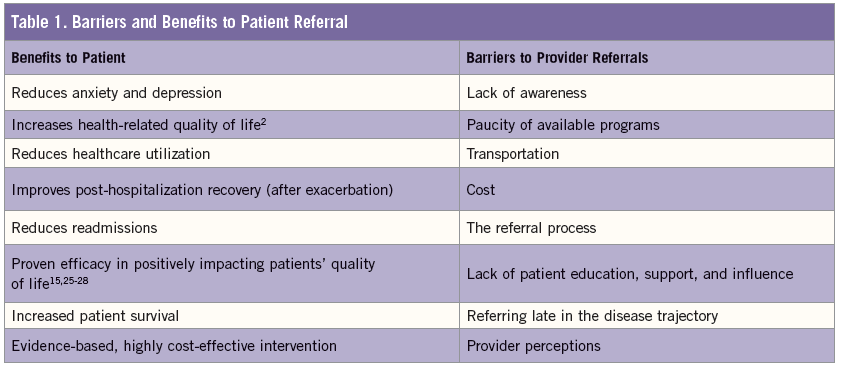
Why is the rate of provider referrals so low? Lack of awareness, paucity of available programs, transportation, cost, and the referral procedure or process all contribute to the pitfalls in PR referral. While many primary care practitioners manage COPD patients, a systematic review found that only 3% to 16% of COPD patients were referred to a PR program, and only 1% to 2% of those referred patients participated.15 Identifying barriers to referring patients early and frequently to PR programs is the key to increasing its implementation (Table). They include:
• Diminished awareness and marred perceptions. A study by Johnston et al looked at expected vs actual implementation of evidence-based guidelines in the management of COPD patients, and barriers and facilitators to implementation for doctors and patients.16 They found that providers expressed a lack of awareness of a PR program, and some admitted that they were unaware it was “usual” care for a COPD patient. Providers who were aware of a PR program said that they did not understand the referral process or thought the time required to make a referral was problematic.16
Concerns over challenges that patients might face in attending a PR program, including transportation and space availability, played a role in whether the provider made the referral.17 Even when the beneficial health effects of PR were recognized, many providers believed that time, travel, and patient effort were costs that outweighed the benefits.18
Other providers noted that the referral process as arduous,18 uncertain as to whether or not they could refer patients,17,19 unfamiliar with patient eligibility requirements,18 and had a lack of knowledge as to where PR fits into the case management of a COPD patient.17
• Lack of patient education, support, and influence. Providers can have a major influence in advocating for and facilitating the patient’s interest in PR—a significant dynamic that should not be underestimated.20 Patients have expressed their unfamiliarity with the multitude of benefits—including the possibility of an improved quality of life9—and did not think PR programs were convenient or important enough to warrant time in their schedules. Further, patients were not interested in participating in a PR program if their provider conveyed that it would be too strenuous or did not believe that it would benefit them.20
• Late referral. Too often providers fail to refer patients earlier in the disease trajectory, generally waiting until symptoms are more severe or after frequent hospitalizations for exacerbations.16 Ironically, a major patient barrier to PR is the patients’ fear that their disease is too severe to participate in and/or benefit from an exercise program.9 Though Medicare only provides coverage for patients with moderate to very severe COPD,21 it remains essential for providers to avoid the pitfall of not referring more patients earlier. Those with moderate grade 2 or grade 3 COPD, for example, should be referred before experiencing a decline in functionality; the perception of severe dyspnea may discourage them.
HOW TO AVOID THE PITFALLS
• Awareness. The 2014 GOLD guidelines provide worldwide evidence-based recommendations for the pharmacological and nonpharmacological management of COPD and identify PR as an important nonpharmacologic intervention. In 2014, the American Thoracic Society and European Respiratory Society published a comprehensive statement describing key concepts and advances in PR. This document cites compelling evidence that establishes PR as a standard of care, outlines the concepts and components of a structured program, and highlights the multitude of benefits.6
It is important that providers identify the local PR program and connect with colleagues who have experienced making referrals.17 Once the practice of referring becomes familiar, it will happen more frequently.16 Support within the healthcare system and community—including referral networks, policy makers, marketing, and funding sources—must exist to aid in raising awareness of PR.15
Providers must also recognize that patients may take some time to see the benefits of a PR program and that a slow introduction to the concept of exercise may have a more immediate positive effect.22
• Early referral. Patients generally do not volunteer complaints of shortness of breath until symptoms are more severe. This makes it difficult for providers to identify and refer patients early enough to minimize resistance to program participation.9,16 Recent data, however, supports that patients with less severe pulmonary obstructions—who participate in PR—have an improvement in outcomes (a score ≥2 on the Modified Medical Research Council dyspnea scale).22 As concluded by Guo and Bruce in their exploration of pulmonary rehabilitation, earlier referrals, in-hospital PR during disease exacerbation, and support of rehabilitation maintenance in the community is an effective means of decreasing healthcare cost utilization and increasing quality-of-life earlier in the disease process.9
• Understanding requirements. Although much of the clinical data that supports intervention involves COPD patients, there are additional lung conditions that warrant a referral to PR. These include interstitial lung disease, persistent asthma, pulmonary hypertension, bronchiectasis, chest wall diseases, and neuromuscular diseases.6 A pulmonary function test and functional capacity test will aid in the assessment and diagnosis of the patient for PR.8
Although Medicare provides coverage for PR, reimbursements vary from plan to plan. Patients should be encouraged to query their insurers regarding covered benefits and patient responsibility.
• Changing provider perceptions. The negative provider perceptions of implementing this intervention will improve with more awareness of the evidence-based impact and value of a comprehensive PR program. Providers should offer patients education, participation support, and persuasive communication necessary to accurately troubleshoot actual versus perceived challenges. The PR programs should provide feedback to referring providers.
PR is an evidence-based standard of care in the management of COPD and other lung diseases, and should be the centerpiece of comprehensive lung disease care. With the economic and social burden that COPD and other lung diseases impose upon the patient and health systems worldwide, management in the primary care arena should be optimized. As the gatekeeper, the primary care provider is in the critical position to educate and promote this intervention for patients with chronic lung disease. ■
Karina M. Berge, MSN, FNP, is a certified family nurse practitioner in the pulmonary rehabilitation program at the University of California, Davis Health System in Sacramento, CA.
Angela Coburn, AAS,RRT, is a respiratory therapist in the pulmonary rehabilitation program at University of California, Davis Health System in Sacramento, CA.
Aimee Kizziar, MHAL, RRT-NPS, is a respiratory therapist in the pulmonary rehabilitation program at University of California, Davis Health System in Sacramento, CA, and also holds a BA in physical education with emphasis in exercise physiology.
Kimberly A. Hardin, MD, is professor of medicine and director of pulmonary rehabilitation at the University of California, Davis Health System in Sacramento, CA.
References:
1. The Link Between Asthma & COPD. American Lung Association. www.lung.org/associations/states/illinois/news/the-link-between-asthma.html. Accessed April 6, 2015.
2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
3. American Lung Association. Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality. March 2013. www.lung.org/finding-cures/our-research/trend-reports/copd-trend-report.pdf. Accessed April 18, 2015.
4. Cancer costs projected to reach at least $158 billion in 2020. National Institutes of Health. www.nih.gov/news/health/jan2011/nci-12.htm. Accessed on April 18, 2015.
5. CDC reports annual financial cost of COPD to be $36 billion in the United States. Chest. July 24, 2014. www.chestnet.org/News/Press-Releases/2014/07/CDC-reports-36-billion-in-annual-financial-cost-of-COPD-in-US. Accessed April 18, 2015.
6. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. Am J Respir and Crit Care Med. 2013:188(8):e11-e40.
7. van Ranst D, Stoop W, Meijer J, et al. Reduction of exacerbation frequency in patients with COPD after participation in a comprehensive pulmonary rehabilitation program. Int J Chron Obstruct Pulmon Dis. 2014;9:1059-1067.
8. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Pulmonary Rehabilitation Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
9. Guo SE, Bruce A. Improving understanding of and adherence to pulmonary rehabilitation in patients with COPD: a qualitative inquiry of patient and health perspectives. PLoS One. 2014;9(10):e110835.
10. Dressendorfer RH. American College of Sports Medicine. Exercise for persons with chronic obstructive pulmonary disease. www.acsm.org/docs/current-comments/exerciseforpersonswithcopd.pdf. Accessed December 12, 2014.
11. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772-778.
12. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331-342.
13. Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793.
14. Hodgkin J, Celli B, Connors G. Pulmonary Rehabilitation Guidelines to Success. St. Louis, MO: Mosby, Inc.; 2009.
15. Johnston K, Grimmer-Somers K. Pulmonary rehabilitation: overwhelming evidence but lost in translation? Physiother Can. 2010;62(4):368-373.
16. Johnston K, Grimmer-Somers K, Young M, et al. Which chronic obstructive pulmonary disease care recommendations have low implementation and why? A pilot study. BMC Res Notes. 2012;5:652.
17. Johnston KN, Young M, Grimmer KA, et al. Barriers to, and facilitators for, referral to pulmonary rehabilitation in COPD patients from the perspective of Australian general practitioners: a qualitative study. Prim Care Respir J. 2013;22(3):319-324.
18. Johnson KN, Young M, Grimmer-Somers KA, et al. Why are some evidence-based care recommendations in chronic obstructive pulmonary disease better implemented than others? Perspectives of medical practitioners. Int J Chron Obstruct Pulmon Dis. 2011;6:659-667.
19. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;8(4):280-290.
20. Keating A, Lee AL, Holland AE. Lack of perceived benefit and inadequate transport influence uptake and completion of pulmonary rehabilitation in people with chronic obstructive pulmonary disease: a qualitative study. J Physiother. 2011;57(3):183-190.
21. Centers for Medicare and Medicaid Services. Your medicare coverage: pulmonary rehabilitation program. www.medicare.gov/coverage/pulmonary-rehab-program.html. Accessed April 2015.
22. Global strategy for the diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease. January 2015. www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed April 2015.
23. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2010.
24. Hardin K, Meyers F, Louie S. Integrating palliative care in severe chronic obstructive lung disease. COPD. 2008;5(4):207-220.
25. Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1):CD005305.
26. Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalization for acute exacerbations of chronic obstructive pulmonary disease: randomized controlled study. BMJ. 2004;329(7476):1209.
27. Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality—a systematic review. Respir Res. 2005;6:54.
28. Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423-428.


