Author:
Bilal Hameed, MD
Citation:
Hameed B. A primary care guide to the diagnosis and management of overt hepatic encephalopathy. Consultant. 2018;58(5):e161.
ABSTRACT: Hepatic encephalopathy (HE), a major complication of cirrhosis, is defined by its spectrum of neurocognitive impairment, which ranges from minimal symptoms (covert HE) to clinically apparent symptoms (overt HE). Patients with overt HE may present with behavioral or cognitive dysfunction and impaired motor function. Given the substantial HE burden, recognizing and managing its precipitating factors (eg, gastrointestinal bleeding, bacterial infection) may improve outcomes. Daily therapy with lactulose, either alone or with the oral nonsystemic antibiotic rifaximin, is recommended to reduce the overt HE recurrence risk. Preventing overt HE recurrence in adults with cirrhosis would reduce disease burden and improve clinical outcomes.
KEYWORDS: hepatic encephalopathy, cirrhosis, liver dysfunction, management, lactulose, rifaximin
Hepatic encephalopathy (HE), a major complication of cirrhosis, is estimated to affect 30% to 50% of patients with liver disease.1-3 HE is a spectrum of neurocognitive impairments ranging in severity from minimal symptoms (covert HE) to overt HE, which can lead to coma in the most severely affected patients.1 Patients with covert HE have neurocognitive impairment that is not clinically apparent but that can be identified via paper-pencil, computerized, or neurophysiologic testing.4 In contrast, patients with overt HE have clinically apparent symptoms, such as impaired motor function (eg, asterixis, hyperreflexia, rigidity) and impaired behavioral or cognitive function (eg, personality changes, aggression or agitation, disorientation).4,5
HE is a common reason for hospitalization in affected patients.6,7 In the United States, hospitalizations related to HE increased from 48,295 in 1993 to 156,205 in 2014.8 Furthermore, the development of HE has been associated with a 1-year mortality rate of 64% and a 5-year mortality rate of 85%.9 Health-related quality of life (HRQOL) is significantly decreased in patients with cirrhosis and HE compared with patients with cirrhosis without HE.10 Most patients with cirrhosis are likely to be under the care of a specialist; however, patients with liver disease, including those with HE, may seek care from their primary care providers.11 Thus, this article provides an overview of the burden of overt HE and the suggested approach to diagnosis and management of the condition in the primary care setting.
METHODS
The PubMed database was searched to identify English-language articles using the following keywords to identify publications available through September 20, 2017, for inclusion in this review: hepatic encephalopathy, epidemiology, diagnosis, management, treatment, dementia, and asterixis. Reference lists from the selected publications were examined for identification of additional articles. Annual hospitalizations in the United States for patients with HE were determined using data from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.8 Patients with HE-related hospitalizations were identified according to the International Classification of Diseases, Ninth Revision, Clinical Modification code for HE (ICD-9-CM 572.2).8
RESULTS
Burden of HE
HE is associated with substantial health care utilization for patients with liver disease.12,13 Although current data are not available, the overall costs for HE-related hospitalizations increased from $4.7 billion in 2005 to $7.3 billion in 2009 (2009 US dollars).12 Economic costs associated with HE-related hospitalizations in the United States increased more than 50% between 2004 and 2010, from $22,511 to $37,598 per discharge.13 The mean duration of HE-related hospitalizations ranged from 6 to 9 days.12-14 One study showed that patients with recurrent HE account for most HE-related hospitalizations: 9% of patients with 2 or more episodes of overt HE within a mean time to recurrence of 72 days comprised 60% of overt HE episodes, of which 88% resulted in hospitalization.15 In an Italian study, most (92.9%) patients hospitalized due to HE had experienced HE recurrence (mean time to recurrence, 96 days).14 Non–hospital-related costs are also burdensome for patients with HE and include outpatient office visits, medications, screenings for complications, and care facilities (ie, nursing home, rehabilitation center).13 Thus, effectively managing ongoing episodes and reducing the risk of overt HE recurrence in patients is imperative for minimizing the economic burden of HE.
In addition to the burden of HE-associated health care costs, HE negatively impacts numerous aspects of patient daily life, including QOL, employment, and finances.10,16-18 In patients with cirrhosis and previous episodes of overt HE, neurocognitive status was negatively associated with HRQOL (P < .05).16 Patients who were aware that they had had a previous episode of HE had a significantly improved HRQOL compared with patients lacking this awareness (P = .04). In patients with cirrhosis and hepatitis C virus infection, HE was negatively associated with general and liver disease–specific HRQOL (P < .001 for both).10 Furthermore, symptoms of depression were significantly associated with increasing severity of HE (no HE, 50%; covert HE, 66.7%; overt HE, 100%; P = .05).10 In 1 US and Italian study, the percentage of patients without a history of overt HE who were employed within the previous year was significantly greater than that of patients with previous overt HE (65% vs 18%, respectively; P = .001).17 These results were consistent with another US study, which also reported that a significantly greater percentage of patients with cirrhosis without a previous episode of HE were employed compared with patients with previous HE (81.0% vs 12.5%; P = .001).18 In addition, a significantly greater percentage of patients with cirrhosis with previous HE had a worse financial status compared with those without previous HE (85% vs 61%, respectively; P = .02).18
The negative impact of HE also extends to a patient’s family and caregivers.16,18,19 According to 2 instruments that assess caregiver burden, patients with cirrhosis with previous HE were considered a significantly greater burden on caregivers compared with patients without previous HE (P = .02 for both instruments).18 For example, the burden of time spent caring for the patient was significantly greater for caregivers of patients with overt HE compared with patients with no HE (P = .007).16 Caregivers have indicated a feeling of being “tied down” related to increased responsibilities (eg, household tasks, finances) and continually assessing patients for symptoms of overt HE.19 A survey of caregivers of patients with cirrhosis indicated that the first episode of HE was unexpected and that they experienced anxiety and a feeling of helplessness.19 However, caregivers noted that this first diagnosed episode of HE helped them to improve their surveillance and assessment of symptoms that suggested HE recurrence.
Patient Population and Risk Factors
The development of HE can occur in patients with acute liver failure, cirrhosis, and portosystemic shunting (ie, spontaneous or transjugular intrahepatic portosystemic shunt [TIPS] placement).1,20 Acute liver failure is a rare condition (approximately 2000 individuals affected annually in the United States) commonly caused by drug toxicity (eg, acetaminophen, dietary or herbal supplements), viral hepatitis, or other conditions (eg, Wilson disease, autoimmune hepatitis, Budd-Chiari syndrome).21-23 Cirrhosis has numerous etiologies, including chronic viral infection (ie, hepatitis B and C virus infection), nonalcoholic fatty liver disease, alcoholic liver disease, cholestatic liver disease (eg, primary sclerosing cholangitis, primary biliary cholangitis), autoimmune liver disease, and metabolic liver diseases (eg, hemochromatosis, α1-antitrypsin deficiency).24-26 Patients with cirrhosis can develop portal hypertension, which can be lowered in severity by TIPS placement between the portal and hepatic veins, which shunts the blood from portal circulation to systemic circulation; because of this liver bypass system, patients with TIPS have an increased risk of developing HE.27 Risk factors associated with the development of HE after TIPS placement include older age, hyponatremia before TIPS placement, higher Model for End-Stage Liver Disease–Sodium (MELD-Na) score, higher total bilirubin level, high creatinine level, low albumin level, and a previous episode of overt HE.28-30
In addition to understanding the underlying pathology of cirrhosis and the potential impact on HE development, health care providers (HCPs) should be aware of the clinically measurable factors that can play a role in precipitating HE (Figure 1).1,4,31 Episodic HE also can occur in the absence of recognized precipitating factors (ie, spontaneous HE).32 Risk factors for the development of HE in patients with cirrhosis include hyponatremia (hazard ratio [HR], 1.4; 95% CI, 1.0-1.8; P = .03), serum creatinine of 1.3 mg/dL or greater (HR, 1.5; 95% CI, 1.1-2.0; P = .007), and serum bilirubin 1.9 mg/dL or greater (HR, 1.9; 95% CI, 1.4-2.6; P < 0.001).33 Conditions such as gastrointestinal bleeding, bacterial infection, electrolyte imbalance, constipation, and diuretic overdose have been shown to precipitate the development of HE (Figure 1).1 In 1 study, the most common precipitating factors identified in patients hospitalized with overt HE were lactulose nonadherence, dehydration, acute kidney failure, constipation, and infections.34 Another study reported that 88% of patients with an HE-related hospitalization had an identifiable precipitant, with spontaneous bacterial peritonitis (SBP; 20.5%), constipation (18.3%), urinary tract infection (UTI; 15.3%), and gastrointestinal bleeding (13.6%) as the most common factors.35 Furthermore, in hospitalized patients with HE, individuals with comorbid infection (ie, UTI, biliary tract infection, sepsis, cellulitis, pneumonia, or SBP) showed a significantly increased risk of 30-day, 30- to 90-day, and 90-day to 1-year mortality compared with patients with HE without a comorbid infection (P ≤ .001 for all).36 Diuretic overdose, which can cause hypovolemia, and HE, which is related in part to a failure to titrate lactulose to the appropriate number of bowel movements, are potentially preventable causes of hospital readmission for patients with decompensated cirrhosis.6 Patients with cirrhosis who were hospitalized without HE or with HE grades 1 or 2 (West Haven criteria) who experienced a worsening in HE severity (ie, to grades 3 or 4) had significantly increased odds of mortality during their hospitalization compared with patients without worsening HE (53% vs 4%, respectively; P < .0001; OR, 27.2; 95% CI, 13.8-53.4).37 Thus, managing (eg, preventing) precipitating factors in patients at risk for HE is warranted.1

Diagnosing HE
The diagnosis of HE includes the exclusion of other potential causes for symptoms and the confirmation of a diagnosis of cirrhosis (Figure 2).5,11,38 The most common symptoms identified by HCPs when establishing a diagnosis of overt HE included confusion (77.5%); altered mental status (57.1%); disorientation to time, place, or person (48.3%); lethargy (46.3%); and asterixis (45.2%).15 Asterixis is a reversible condition that affects motor control in the extremities, often resulting in symmetric hand “flapping” (Figure 3).39 Asterixis is not unique to HE,39 and clinical evaluation of a patient includes the assessment of the extremities affected and the evaluation for asterixis as the patient holds various positions.39,40 Some patients with neurologic dysfunction may have mostly normal liver test results, which may result in an HCP missing a diagnosis of cirrhosis.38 For these patients, ultrasonography, computed tomography, or magnetic resonance imaging of the liver or transient elastography (FibroScan, Echosens, Paris, France) could be useful for diagnosing cirrhosis and could help identify portosystemic shunting.38,41


Cognitive impairment is not unique to HE and can also occur with dementia or other neurologic conditions and is a part of the normal aging process.42,43 Thus, a diagnosis of HE is considered when other conditions, including dementia or normal aging processes, have been excluded.1,42 Primary care providers should consider having the patient with suspected HE consult with a specialist to confirm the HE diagnosis.11 The West Haven criteria, which are based on clinical criteria and severity of symptoms, are generally used to evaluate the cognitive status of patients suspected or known to have HE.1,15 However, the Hepatic Encephalopathy Scoring Algorithm (HESA) was developed to improve the objectivity of the West Haven criteria by including both clinical judgment and neuropsychological outcomes.44,45 More recently, the HE Grading Instrument (HEGI) was introduced as a simplified, standardized approach that HCPs may find useful in clinical practice for identifying patients with overt HE and includes a caregiver e-diary that can be used as a complementary tool for evaluating symptoms of overt HE.5 The HEGI considers 1 or more of the following criteria sufficient for a diagnosis of overt HE: disorientation, both lethargy and asterixis, or coma.5 With a diagnosis of HE, the condition can be subcategorized by the frequency of occurrence: episodic HE (1 episode in ≤6 months), recurrent HE (>1 episode in ≤6 months), and persistent HE (no resolution of symptoms).1,4
Serum ammonia may be helpful for initial evaluation of patients with altered mental status when a diagnosis of liver disease has not been established.46 Arterial ammonia levels 146 µmol/L or greater have been associated with cerebral edema in patients with acute liver failure.47 However, no relationship has been shown between elevated serum ammonia levels and severity of HE in patients with cirrhosis.48 Measurement of serum ammonia levels is not indicated for the diagnosis of HE in routine clinical practice; furthermore, accurate measurement of serum ammonia levels is dependent on the provider ensuring sample collection without the use of a tourniquet and rapid analysis (≤20 minutes) following sample collection.49 In addition, multiple causes of hyperammonemia are not related to the liver, such as gastrointestinal bleeding, kidney failure, hypovolemia, urea cycle disorder, and parenteral nutrition.50,51
Symptoms of HE can resolve with or without treatment and might not be directly observed by HCPs but rather by family members or caregivers of patients.5 When evaluating a patient with suspected overt HE, physicians with extensive expertise in the care of patients with HE noted that they actively sought caregiver input regarding patient daily life (95%), mental status (57.5%), sleep habits (32.5%), adherence to treatment (22.5%), personality changes (22.5%), precipitants for overt HE (15.0%), and timing of symptoms (12.5%).5 Caregivers play an important role in the management of patients with HE; in 1 study, 83% of caregivers reported contacting HCPs only when symptoms of an acute episode of overt HE worsened or did not resolve.5 In another study, most episodes of HE were recognized as such by HCPs; however, 25% of HE episodes were identified by caregivers.15 Thus, the importance and value of caregivers as active partners in the management of patients with HE should not be overlooked.19
Management of HE
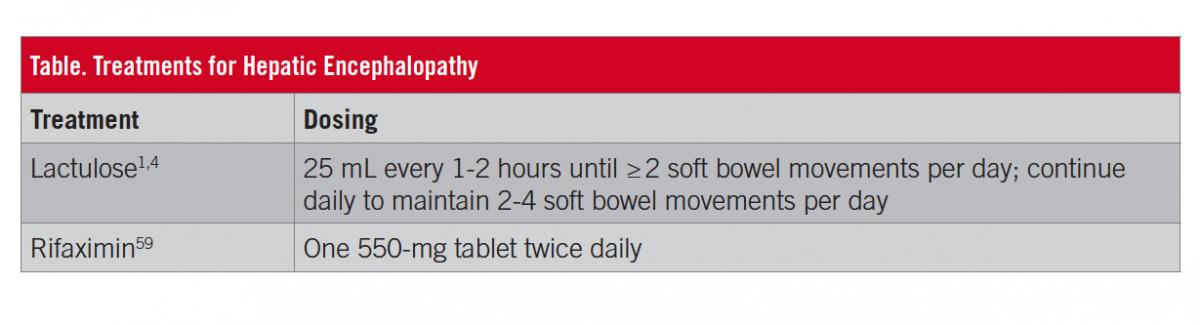
Ultimately, the severity of HE symptoms impacts and drives the rates of hospitalization, the overall costs for affected patients, and the burden of the condition. Therefore, treatment of an overt HE episode and reduction in the risk of recurrence are critical. The initial goals of treatment for patients with a diagnosis of overt HE are to treat which if any precipitating factor(s) might be involved and to improve mental status; reducing the risk of overt HE is recommended after the acute episode of HE resolves.1,11 However, learning impairment may persist in patients with overt HE, regardless of treatment.52 First-line treatment for many patients experiencing an episode of overt HE is lactulose, a nonabsorbable disaccharide that acts to metabolize colonic bacteria, in turn decreasing colonic pH and the survival of urease-producing gut bacteria and increasing fecal nitrogen waste.53-55 For preventing overt HE recurrence, guidelines from the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver recommend lactulose alone or in combination with rifaximin.1 Lactulose is an oral syrup administered daily that is titrated to maintain 2 to 4 soft bowel movements per day (Table).1,4 Patients with more-severe HE (ie, West Haven criteria grade 3 or 4) are likely to be hospitalized; for patients unable to swallow an oral syrup, lactulose can be administered via nasogastric or nasojejunal tube, or rectally.4,56 Lactulose use is associated with gastrointestinal-related adverse effects, including flatulence, abdominal discomfort, and diarrhea.4 The overuse of lactulose may cause dehydration, hypernatremia, and, ironically, increased risk of HE.1 The adverse effects of lactulose therapy have been associated with nonadherence to treatment, which, in 1 study, was significantly associated with recurrence of HE (P = .00001) within a mean of 3 months.57 In 1 study, polyethylene glycol 3350 solution, which is commonly used as a bowel preparation for colonoscopy, significantly improved the severity of HE at 24 hours for patients hospitalized with HE (P = .002).58
Rifaximin is an oral nonsystemic antibiotic indicated for reducing the risk of recurrence of overt HE in adults (Table)59; however, a comprehensive review of clinical trials supports that rifaximin also improves symptoms in patients experiencing an acute episode of overt HE.60 The short-term (ie, 6 months) and long-term (ie, ≥2 years) efficacy and safety of rifaximin have been demonstrated in patients with a history of recurrent overt HE.61,62 Compared with placebo, rifaximin significantly decreased the risk of recurrence of overt HE by 58% (P < .001; number needed to treat [NNT], 4) and the risk of HE-related hospitalizations by 50% (P = .01; NNT, 9) during 6 months of treatment.61 Concomitant lactulose use was reported by most (approximately 91%) patients in each group. In rifaximin vs placebo groups, adverse events (≥10% of patients) included peripheral edema (15.0% vs 8.2%, respectively), nausea (14.3% vs 13.2%), dizziness (12.9% vs 8.2%), fatigue (12.1% vs 11.3%), ascites (11.4% vs 9.4%), diarrhea (10.7% vs 13.2%), and headache (10.0% vs 10.7%). Clostridium difficile infection was reported in 2 patients who received rifaximin; however, both patients were at increased risk (eg, recent hospitalizations with antibiotic treatment) and were able to continue rifaximin therapy during treatment for the C difficile infection.
Long-term (≥2 years) open-label rifaximin treatment of patients with a history of recurrent overt HE (including newly enrolled patients and patients previously enrolled in a randomized study61) experienced a lower rate of HE-related and all-cause hospitalizations vs the historical population that received placebo in the randomized trial (HE-related: 0.21 events/person-years of exposure [PYE] vs 0.72 events/PYE, respectively; all-cause hospitalization: 0.45 events/PYE vs 1.3 events/PYE, respectively).62 In this study, 89.8% of patients received concomitant lactulose. In the long-term, open-label study, C difficile infection occurred in 6 patients, all of whom had multiple risk factors for C difficile infection; these infections resolved after antibiotic treatment.62
In another study, the mean number of emergency department admissions during 1 year was significantly improved during treatment with rifaximin compared with before treatment (2.1 vs 1.6, respectively; P = .001); furthermore, the mean length of hospitalization per admission was significantly improved (13.5 days vs 8.6 days; P = .02), resulting in an estimated annual decrease of 31%.63 In this study, most (86.2%) patients received concomitant lactulose.63 Previously, oral neomycin and metronidazole were therapeutic options for managing patients with HE; rifaximin is currently the antibiotic preferred by providers given concerns related to adverse effects and toxicity with systemic antibiotics.
Malnutrition affects most patients (up to 80%) with cirrhosis to varying degrees.64 Dietary protein restriction is not recommended for the management of HE, since it can exacerbate protein-calorie malnutrition and loss of skeletal muscle.1,65 Furthermore, administration of a normal-protein diet in patients with HE did not change the course of HE compared with those on a low-protein diet.65 In patients with HE, protein intake of 1.2 to 1.5 g/kg per day is recommended by the American Association for the Study of Liver Diseases.1
Liver transplantation is the final treatment option for patients with HE, and is considered when patients experience poor hepatic function.1 A single-center retrospective analysis showed no significant difference in survival 1, 3, and 5 years posttransplant between patients with HE grades 1 and 2 vs grades 3 and 4 (according to West Haven criteria; P = .75).66 However, patients with HE grades 3 and 4 were more likely to experience HE sequelae (eg, impaired memory, dysarthria) following transplant compared with patients with less-severe HE (grades 1 and 2; P = .05).66 Thus, preventing patients with less severe HE from progressing to more-severe forms of HE may improve posttransplant outcomes.66
CONCLUSIONS
HE is a major complication of cirrhosis associated with a substantial disease burden that includes increased health care utilization (eg, hospitalizations) and negatively impacts both patient daily life and that of their families and caregivers.6,7,10,12,13,16-19 Overt HE is characterized by a spectrum of neurocognitive symptoms ranging from impaired behavioral or cognitive function to, in its most severe form, coma.11 Family and caregivers can serve as important resources for HCPs in the early identification of symptoms suggestive of HE. However, other medical conditions may exhibit symptoms similar to those seen with HE.5,11,38 Understanding the symptoms, variability in presentation, and precipitating factors associated with the development of overt HE is important in the early diagnosis and successful management of the condition. Management of HE includes the treatment of precipitating factors, treatment (eg, lactulose, rifaximin) to improve mental status in patients experiencing an acute episode, and reduction of the risk for overt HE recurrence (eg, lactulose, rifaximin) in those with a history of overt HE.1,11 For patients who require liver transplant, outcomes (ie, survival, no sequelae) may be improved with more-intensive management prior to transplant.66
Bilal Hameed, MD, is an associate professor of medicine, director of the Transplant Hepatology Fellowship, and associate clinic chief, Hepatology, at the University of California–San Francisco in San Francisco, California.
DISCLOSURES:
Technical editorial assistance was provided, under the direction of the author, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, at Synchrony Medical Communications LLC, West Chester, Pennsylvania. Funding for this support was provided by Salix Pharmaceuticals, Bridgewater, New Jersey.
Dr Hameed reports receiving research support from GENFIT, Gilead Sciences Inc, Conatus Pharmaceuticals Inc, and Intercept Pharmaceuticals Inc.


