Pitfalls of Venous Thromboembolism
There are few situations that cause more dread among clinicians than missing the diagnosis acute pulmonary embolism (PE). This is due in no small part to the difficulty interpreting nonspecific signs and symptoms from PE and failure to properly assess risk factors for venous thromboembolism (VTE). Recent evidence casts doubt on the dogma that PE is always a complication of peripheral deep venous thrombosis (DVT).
Approximately one-third of patients with symptomatic VTE manifest PE whereas two-thirds manifest DVT alone.1,2 DVT and PE appeared to be distinct, albeit overlapping, clinical entities with different natural histories. Whether underdiagnosed and not treated, or overdiagnosed and treated, PE remains affects an estimated 500,000 persons annually in the United States alone.3-6
________________________________________________________________________________________________________________________________________________________________________________________________________________________
RELATED CONTENT
Bridge Therapy for Venous Thromboembolism
Can NSAIDs Increase VTE Risk?
______________________________________________________________________________________________________________________________________________________________________
This column highlights pitfalls during the diagnostic evaluation for PE and discusses how to avoid them to minimize morbidity and mortality. We revisit Virchow’s Triad and introduce Murin’s Rule. Best practice recommendations can be found in the most recent American College of Chest Physicians (ACCP) guidelines.3,4

Natural History of Acute PE
Acute PE is associated with high mortality ranging between 11% to 23%5 from cardiovascular collapse or fatal cardiac arrhythmias. The hemodynamic consequences of PE range from acute pulmonary hypertension (PHTN), acute on chronic PHTN, or potentially lethal obstructive shock. Despite anticoagulant therapy, VTE has been reported to recur frequently in the first few months after the initial diagnosis—with a recurrence rate of 7% at 6 months. Death occurred in 6% of DVT cases and 12% of PE cases within 1 month of diagnosis.1
The challenge for every clinician is not to miss the diagnosis of PE. A major pitfall is to evaluate a patient suspected of PE without a disciplined strategy and objective evidence with risk stratification. Relying on symptoms, signs, chest X-ray, arterial blood gases, and electrocardiogram is unreliable as none are specific enough for diagnosis of PE. The classic triad of dyspnea, chest pain (pleurisy), and hemoptysis is uncommon—occurring 20% or less in patients with proven PE.6 Patients may not experience symptoms or complain of them for days or even weeks before presentation to a clinic or hospital for evaluation. PE untreated is associated with mortality up to 6-fold higher than reported with anticoagulant treatment (3% to 8%).6
 Risk Factors for VTE
Risk Factors for VTE
Risk factors from the patient history can assist in the important assessment of clinical pre-test probability (CPTP) and help determine the best approach to making or excluding the diagnosis of PE.
Failure to recognize the clinical risk factor(s) that promote Virchow’s triad of hypercoagulability, blood vessel wall injury, and venous stasis is another common pitfall (Table 1).7 Some of the risk factors, including advanced age, cancer, and underlying cardiovascular disease, are strongly associated with early mortality.
Overall, 25% to 50% of patient with first-time VTE have no readily identifiable risk factor. This group of patients is categorized as having unprovoked PE. Unprovoked PE is associated with higher rate of recurrence and thus differing treatment recommendations.
Murin’s Rule and Clinical Pre-Test Probability (CPTP)
Signs and symptoms are non-specific in acute PE and lack sensitivity since they overlap with acute exacerbations of COPD, asthma attacks, acute pneumonia, pulmonary aspiration, and congestive heart failure. We have often heard patients with confirmed acute PE report unexplained apprehension and a fear of death, a sense of impending doom, but that observation lacks sufficient sensitivity and specificity. However, the presence of unexplained signs and symptoms can heighten suspicion and begin a diagnostic evaluation that can be guided by Murin’s Rule and disease specific clinical decision rules.
The higher your level of suspicion of the disease or the worse the potential consequences of missing the diagnosis, the higher the level of evidence you should require in order to exclude that disease from your differential.
The most common symptoms in patients with PE and no pre-existing cardiac or pulmonary disease are dyspnea (73%), pleurisy (66%), cough (37%), and hemoptysis (13%).8 However, these symptoms are also very prevalent in patients without PE.9 The combined use of CPTP and either non-invasive or invasive imaging studies increases the chances of appropriately diagnosing or ruling out PE.
(Visit the next page for Clinical Decision Rules in Diagnosing PE)
Clinical Decision Rules in Diagnosing PE
It is necessary to quickly assess whether PE is likely or unlikely in a patient who presents with signs or symptoms suggestive of PE based on CPTP. Such an approach can improve diagnostic accuracy, minimize morbidity, and even prevent death. A common pitfall is not taking enough time in the beginning to calculate CPTP to determine the need for anticoagulation and further imaging studies, including CT pulmonary angiography (CTPA) or ventilation/perfusion (V/Q) scanning.
Two clinical decision rules or scoring criteria can be used to objectify the risk or CPTP for PE: the Wells score (Table 2) and the Geneva score.10,11 The simplified Wells and revised Geneva score assign only 1 point to each risk factor or clinical finding without loss of diagnostic accuracy.11,12 A prospective cohort study, the Pometheus study, compared all 4 clinical rules (including the simplified Wells scoring) in the diagnosis and management of PE to demonstrate the safety of this approach; the 3-month VTE recurrence rates were between 0.5% and 0.6% in low-risk patients with normal d-dimer.13 Note: While we prefer the simplified Wells score, others may favor the Geneva score. The pitfall take-away is to not use one or the other to determine the next course of action in evaluating a patient with suspected PE.
Use either Wells or Geneva score to determine whether PE is likely or unlikely. If suspected PE is unlikely based on low CPTP and fibrin D-dimer is normal, no further diagnostic testing is necessary. These patients have a 3-month recurrence rate of VTE of 0.5%.14
If suspected PE is likely based on high CPTP, the patient in question should promptly be treated with anticoagulants to prevent further venous thrombosis and undergo confirmatory imaging testing—usually CTPA or pulmonary angiography. Pulmonary angiography was long considered the gold standard diagnosis of PE but has largely been supplanted by CTPA. However, a strategy employing this diagnostic imaging cannot diagnose or entirely exclude PE in all suspected cases. Pulmonary angiography if negative for PE is associated with a 1.6% incidence of VTE in the following year.15

Diagnostic Tests
• Fibrin D-dimer is a degradation product of cross-linked fibrin. D-dimer levels are nearly always elevated in VTE and PE. Consequently a normal D-dimer level has a negative predictive value of approximately 95% for excluding VTE or PE when the CPTP is low.10,16 A normal D-dimer level effectively rules out PE in a patient with low CPTP with a 3-month VTE risk between 0% and 0.5%.14,17
Among patients with an intermediate CPTP, a negative D-dimer test result would exclude PE with reasonable negative predictive value only if D-dimer is measured by the ELISA method.16 D-dimer testing should not be obtained in patients with high CPTP as it might mislead and delay anticoagulant treatment and diagnosis.
It is important to realize that there are conditions other than VTE or PE that increase D-dimer levels, including advancing age, trauma, recent surgery, pregnancy, liver disease, malignancy, and disseminated intravascular coagulation.18 Imaging studies are more often necessary to obtain the higher level of evidence needed to diagnose or exclude PE in older patients.
Multidetector CTPA and V/Q scanning are the standard diagnostic imaging studies for PE. CTPA has almost replaced V/Q scanning as a screening test and conventional pulmonary angiography as the reference standard for the diagnosis of PE.19
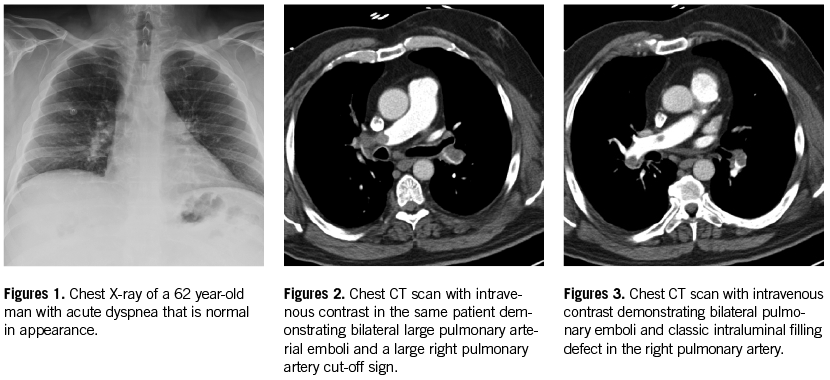
• CTPA is the diagnostic test of choice to confirm or exclude PE as a diagnosis in hospitals who have this technology available. It was confirmed to have high sensitivity (90%) and high specificity (95%) in the PIOPED II trial.20 Its positive predictive value (PPV) varies with the extent and location of the thrombi, ranging from 97% with main or lobar artery clots, 68% with segmental clots, and only 25% with isolated subsegmental artery clots (Figures 1-3).
The 3-month risk for VTE for patients with suspected PE and negative or “normal” CTPA is 1%. However, indeterminate CTPA occur in up to 10% of cases, which presents clinicians with a conundrum. Further testing is needed as VTE can be found in 16% of patients with indeterminate CTPA, per a meta-analysis.21
CTPA is also useful for risk stratification in hemodynamically stable patients by examining for radiographic signs of right ventricular strain and the location of the thrombi.22
Important risks and complications from CTPA include radiation exposure, contrast-induced nephropathy, and allergic reactions to contrast. A substantial number of CTPA could be avoided by adhering to the algorithm employing CPTP and D-dimer testing first.23 It is imperative that the use of CTPA should be limited to those patients with a clear indication—that is, patients with a moderate to high CPTP or patients with an elevated D-dimer in whom PE cannot be excluded without a higher level of evidence.
• V/Q scanning is a test that involves the simultaneous scintigraphic imaging of the pulmonary arteries and airways with much lower radiation dose than CTPA. The interpretation is made based on finding lobar and segmental V/Q mismatch. It is helpful when the result is interpreted into normal or high probability. However, the majority of V/Q scans tend to be intermediate in probability as found in the PIOPED trial.24
Recently, the clinical utility of V/Q scans was revisited in the PISAPED study,25 which proposed the use of perfusion scans only—without ventilation scans and in conjunction with CPTP and CXR findings—for the evaluation of PE. Applying PISAPED criteria to subjects in PIOPED II study, PPV of V/Q scan findings was 80% and NPV was 97%, which are comparable to CTPA in the same population.26
Based on the current available data, V/Q scanning and CTPA are comparable as diagnostic tests for acute PE. The tests should be ordered based on local experience and expertise, availability at each institution, and whether a patient has any contraindications to the alternative imaging test (eg, acute renal failure and iodine allergy).
Risk Stratification
Each patient should be stratified quickly after the diagnosis acute PE is confirmed. Clinical manifestations may range from minimal symptoms to frank shock. Anticoagulant therapy should have been started before imaging studies and can continue if absolute contraindications do not exist. Patients can be divided into 1 of 3 main categories: massive, submassive, and low-risk PE.27
Massive PE is defined as acute PE with sustained shock or hypotension. Submassive PE is defined by acute PE without systemic hypotension, but with either RV dysfunction or myocardial necrosis that could be suggested on echocardiogram, ECG, cardiac enzymes, and brain natriuretic peptide.
Patients with massive or submassive PE should be admitted to the intensive care unit for monitoring and treatment, possibly with thrombolytic therapy. For those who fail thrombolytic therapy or where this therapy is contraindicated, catheter-assisted thrombus removal and surgical pulmonary embolectomy may be warranted but this is dependent on available expertise at the institution.
Treatment of Acute PE
The treatment of choice is systemic anticoagulation with heparin to prevent new thrombosis. This should be initiated as quickly as possible when no absolute contraindication exists, in some cases before the diagnosis has been established
The ACCP guidelines recommend empiric anticoagulation if 1) CPTP is high, 2) CPTP is moderate and testing will not be done within four hours, or 3) CPTP is low and testing will be delayed for over 24 hours.3 Anticoagulation should be started as soon as feasible after the diagnosis has been established, if not already started empirically. A recent study confirmed that early anticoagulation is associated with reduced mortality.28 The only time that anticoagulation may be interrupted is when the patient develops serious bleeding events or in need of invasive procedures or surgery for life-threatening disease. Anticoagulation should be resumed as soon as possible.
Intravenous unfractionated heparin (UFH) should be used in unstable patient or patient with anticipated procedures as it has the shortest half-life (between 1 to 2 hours) and can be promptly reversed with protamine sulfate if necessary. The activated partial thromboplastin time (aPTT) should be measured 6 hours after a IV bolus dose of heparin, and the continuous IV dose should be adjusted to the hospital’s therapeutic range (typically 1.5 times the patient’s baseline aPTT).
If the patient has no anticipated procedures, low molecular weight heparin (LMWH) or fondaparinux are preferred. Vitamin K antagonist (VKA)—eg, coumadin—should be initiated the same day as parenteral therapy with a target INR of 2 to 3. Heparin or LMWH can be discontinued after a minimum of 5 days and until the INR reaches therapeutic levels for at least 24 hours to avoid acquired protein C deficiency that can lead to coumadin skin necrosis. LMWH is recommended over VKA therapy in patients with cancer.29
The minimal duration of treatment is 3 months, which is recommended in patients with provoked PE with transient reversible risk factors, such as trauma, surgery, and significant immobility.3 Extended to indefinite anticoagulation is recommended in patients with unprovoked PE, recurrent PE, high risk for recurrent PE, and patients with active cancer. Risk factors for recurrent PE include a history of cancer, diseases associated with thrombophilia, family history of VTE, symptomatic PE and elevated D-dimer levels after discontinuing anticoagulation.30
The 2012 ACCP guidelines do not include recommendations for all of the available new oral anticoagulants available for the treatment of VTE. Review of recent literature for factor Xa inhibitors rivaroxaban and apixaban provide evidence for noninferiority in prevent recurrent VTE with LMWH and coumadin; apixiaban significantly reduced major bleeding events compared to LMWH and coumadin.31 Extended to indefinite anticoagulation with aspirin or one of the new oral anticoagulants after initial therapy for VTE may significantly reduce recurrence of VTE in selected patients.32,33 It is essential to remember that all anticoagulants, regardless of their mechanism of action, increase the risk of bleeding and can cause serious, potentially fatal bleeding.
Inferior vena cava (IVC) filters can be considered if the patient develops a serious contraindication to anticoagulation treatment (eg, life-threatening bleeding). IVC filter should be removed, if possible, once no longer indicated. IVC filters can be associated with worsening DVT as they increase venous stasis. In addition, there are other complication associated with IVC filter such as filter migration, filter fracture, and procedural-related complications.
Narin Sriratanaviriyakul, MD, is a senior fellow in the division of pulmonary, critical care and sleep medicine at the University of California, Davis.
Susan Murin, MD, is a professor of medicine and the chief of the division of pulmonary, critical care, and sleep medicine at the University of California, Davis.
Samuel Louie, MD, is a professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of California, Davis.
References:
1.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-8.
2. Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002;88(3):407-414.
Additional references at www.consultant360.com.


