Pitfalls in the Initial Management of Mechanical Ventilation: COPD and Asthma
Invasive mechanical ventilation (MV) is a common lifesaving intervention for critically ill patients who are in respiratory failure due to a wide variety of etiologies. With nearly 800,000 patients requiring MV per year and a mounting shortfall of intensivists in the United States, MV management is increasingly falling to internists, family practice providers, emergency department (ED) providers, and others without primary training in advanced MV.1,2
A detailed understanding of the underlying physiologic derangements that are unique to each patient and that lead to endotracheal intubation and MV is required in order to maximize the benefits and minimize the potential harms of MV. In this review, we will provide pragmatic recommendations for the early postintubation MV management period of chronic obstructive pulmonary disease (COPD) and asthma for nonintensivist providers in the ED and intensive care unit (ICU). In a subsequent installment of Pulmonary Pitfalls, we will address acute respiratory distress syndrome and hypermetabolic syndromes such as sepsis.
Risks and Benefits of MV
Regardless of the indication for the use of MV, its benefits in respiratory failure need to be judiciously balanced with the known risks. The delivery of MV with injurious tidal volumes, excessive airway pressures, inadequate end-expiratory pressure, and unmatched patient respiratory demand can lead to volutrauma (alveolar overdistension leading to inflammation and iatrogenic lung injury), barotrauma (pneumothorax and/or pneumomediastinum), atelectrauma (damage from repetitive opening and closing of alveoli), and patient-ventilator asynchrony (PVA), respectively.3,4 Collectively, these insults can lead to ventilator-induced lung injury, which has been associated with increased morbidity and mortality in patients with acute respiratory failure.4 Furthermore, PVA is also a major source of patient discomfort in the ICU and may contribute to increased sedation requirements, longer time on MV, and longer ICU lengths of stay.5 As such, avoiding common pitfalls in the management of MV stands to improve outcomes and experiences of critically ill patients with acute respiratory failure requiring MV (Table 1).

COPD and MV
The fundamental pathophysiologic derangement in COPD is expiratory flow limitation with resultant dynamic hyperinflation (Figure 1), leading to increased work of breathing and ventilation-perfusion mismatch.6 High intra-alveolar pressure from hyperinflation leads to a large pressure gradient between the alveoli and the airway opening (mouth or endotracheal tube) at the end of exhalation (referred to as auto-positive end-expiratory pressure [PEEP] or intrinsic PEEP), requiring high work of breathing to overcome auto-PEEP and produce inspiratory flow (Figure 2). Dynamic hyperinflation results in flattening of the diaphragm and mechanical distortion of the intercostal muscles, both of which lead to a mechanical disadvantage, dyspnea, fatigue, and then respiratory failure.7 Hyperinflation due to expiratory flow limitation also results in regional alveolar distention that can increase physiologic dead space ventilation, with apparently paradoxical worsening of arterial carbon dioxide levels (Paco2) despite a high measured minute ventilation.8 Finally, high intrathoracic pressures from dynamic hyperinflation can result in hemodynamic instability from decreased venous return and even cardiovascular collapse if high pressures disrupt alveolar or airway wall integrity, resulting in tension pneumothorax.9

Figure 1. A chest radiograph demonstrating severe hyperinflation in a patient with asthma. Note the large rib spaces, the more than 10 visible ribs, and the vertically oriented heart.
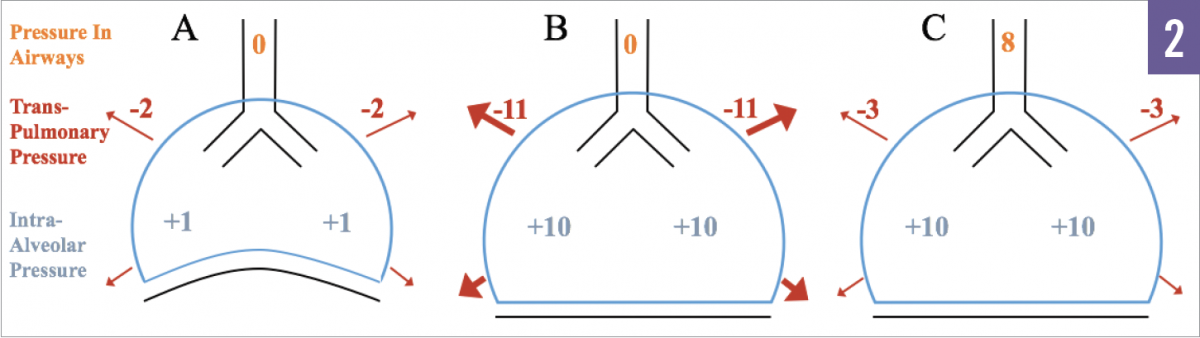
Figure 2. Example A illustrates a healthy patient without hyperinflation. Example B depicts hyperinflation without positive pressure ventilation. Example C depicts hyperinflation with positive pressure ventilation. Example B shows the large transthoracic pressure that is needed to generate inspiratory flow. In example C, the addition of 8 cm H2O PEEP decreases the gradient, requiring less transthoracic pressure, and hence less work, to initiate inhalation.
Several strategies for the initial management of MV may help to mitigate complications and serve to counteract the pathologic derangements of COPD. In this regard, 2 concepts deserve mention—namely, that allowing for adequate time to exhale will improve dynamic hyperinflation, and that MV should not be adjusted to normalize arterial pH and Paco2 but to prevent hyperinflation and its complications. Low tidal volumes (6-8 mL/kg of predicted body weight) may help to avoid volutrauma and decrease dynamic hyperinflation by reducing the amount of volume that needs to be exhaled with each breath.4 The respiratory rate should be adjusted so that breaths initiate at the end of the prolonged exhalation when expiratory flow is at or near zero on the ventilator’s flow-time curve (Figure 3). Further increasing the respiratory rate will lead to breath stacking and increasing hyperinflation, which may increase physiologic dead space and worsen Paco2 despite the increase in calculated minute ventilation.7

Figure 3. In severe obstruction, the exhaled volume (TVe) may be less than the inspiratory volume (TVi), leading to incomplete exhalation, progressively increasing lung volumes, and auto-PEEP (ie, breath stacking). In a normal, unlabored breath, flow and volume should return to zero (functional residual capacity) prior to initiation of the next breath.
Achieving relative hypopnea often requires the adjunctive use of narcotic analgesic and/or sedative medications to limit respiratory distress and patient discomfort. In patients with severe obstruction and persistent expiratory flow limitation despite optimizing the respiratory rate, decreasing the set inspiratory time (I-time) may allow more time for exhalation.
It is important to note, however, that shortening the I-time decreases the time over which mechanical support is provided and may decrease MV support intended to offset the work of breathing. Furthermore, when appreciably shorter than a patient’s neural I-time set point (the intrinsic I-time desired by a patient), decreasing the ventilator’s set I-time may result in considerable respiratory distress and PVA, making it more difficult to achieve tidal volume and respiratory rate goals and subsequently potentially worsening dynamic hyperinflation.
In patients with severe obstruction, it is frequently difficult to eliminate all hyperinflation even with the use of tidal volume limitation, relative hypopnea, and I-time optimization and so, as mentioned above, the goal of MV should not be normalization of pH and Paco2, but rather maintenance of hemodynamically inconsequential pH (typically ≥7.2 for most patients) while supporting the work of breathing, providing adequate oxygenation, and waiting for clinical improvement (a strategy commonly referred to as “permissive hypercapnia”).9 Especially in patients with chronic respiratory acidosis, the goal of ventilation is not a carbon dioxide level in the normal range.10,11
The application of PEEP in the setting of COPD is poorly studied, but it is generally agreed upon that all patients undergoing MV should receive some minimal amount of PEEP (eg, ≥5 cm H2O). Dynamic hyperinflation results in persistent auto-PEEP, even in the absence of ventilator-applied (extrinsic) PEEP. Auto-PEEP mediates the increased work of breathing, ventilation-perfusion mismatch, and hemodynamic compromise as noted above, and the application of increasing amounts of extrinsic PEEP has a number of theoretical benefits. Extrinsic PEEP can decrease the work of breathing by reducing the gradient between the relatively low pressure in the ventilator circuit and the relatively high end-expiratory pressure in the alveoli that occurs in the setting of hyperinflation, resulting in a decrease in the amount of work required of a patient to overcome the ventilator’s trigger threshold (Figure 2). In addition, extrinsic PEEP is thought to “stent” open some airways, preventing dynamic airway collapse on exhalation.9
Note that airflow obstruction is not homogeneously distributed throughout the lung, and multiple mechanisms of airflow limitation may occur simultaneously (eg, airway edema, bronchospasm, mucoid impaction, dynamic expiratory collapse).6 As a result, the effects of applying extrinsic PEEP are not always predictable, and so pH and carbon dioxide should be monitored frequently during ventilator changes to ensure that anticipated physiologic effects are observed.
In addition, the fraction of inspired oxygen (Fio2) should be rapidly decreased to avoid oxygen toxicity, given the fact that patients rarely demonstrate severe hypoxemia.6 The breath initiation trigger should be flow-triggered for patient comfort and set to the lowest sensitivity that avoids auto-triggering, due to a high incidence of failed triggers in patients who have COPD (Figure 4).6,12,13
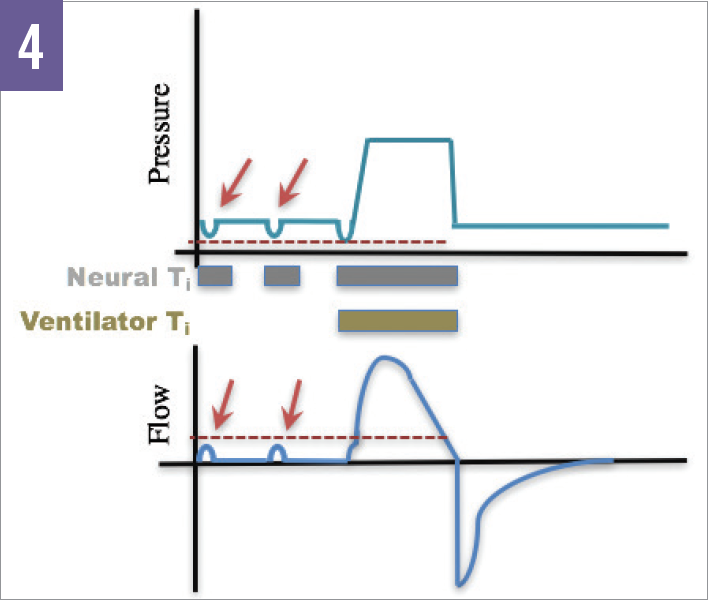
Figure 4. Ineffective or “failed” triggers occur when the patient attempts a breath but cannot meet the predetermined pressure or flow threshold for the ventilator to trigger a breath.
Asthma and MV
Patients with status asthmaticus develop respiratory failure due to airflow limitation resulting from airway edema, bronchospasm, and often severe small-airway mucous plugging. While the delivery of MV in status asthmaticus generally follows principles similar to those in COPD, the often severe, diffuse small-airway involvement can make MV particularly challenging in these patients.
Overcoming high airway resistance in severe asthma with positive pressure MV often leads to high measured peak inspiratory pressures (often >50 cm H2O). The large amount of pressure required to deliver a breath is testament to the incredible amount of work required of patients with asthma in exacerbation. This should be considered before initiating neuromuscular blockade. In asthma, however, peak pressure does not necessarily reflect alveolar pressure when airway resistance is high, and in this regard, peak pressure has not been shown to predict risk of pneumothorax.9 Instead, management of MV is aimed at limiting the transpulmonary pressure (alveolar pressure minus intrapleural pressure), which is the distending pressure “felt” by the alveoli and distal small airway walls.9 Since intrapleural pressure is not routinely measured, the plateau pressure measured after a brief end-inspiratory pause is used as a surrogate for the transpulmonary pressure. While lower plateau pressures are always preferred, maintaining plateau pressure below 25 to 30 cm H2O will generally limit the risk of barotrauma and hyperinflation.14
Hyperinflation is often severe in asthma and leads to auto-PEEP frequently in excess of 15 cm H2O. As with COPD, providing additional extrinsic PEEP with the ventilator to “match” the auto-PEEP and to aid expiratory flow has not been studied extensively, and thus an optimal strategy remains unclear. In most patients, PEEP should be maintained at 5 cm H2O and, if a trial of increased levels of extrinsic PEEP is undertaken, extrinsic PEEP should be limited to no more than 80% of measured auto-PEEP, and plateau pressure and blood gases should be monitored frequently to prevent worsening of hyperinflation and ventilation, respectively.9
Instead, to decrease hyperinflation, MV should be adjusted to maximize exhalation time. The most efficient method of improving dynamic hyperinflation is to decrease the respiratory rate (eg, 10-14 breaths/min, titrated to the highest respiratory rate that allows near-complete exhalation) and tidal volume (6-8 mL/kg ideal body weight).9,15 The use of narcotic analgesics and/or sedation is often required to blunt the patient’s respiratory drive to achieve these goals.
If patients continue to have severe auto-PEEP despite deep sedation, a brief period of neuromuscular paralysis is recommended to allow for a PEEP titration and a clearer assessment of hyperinflation.9 Shortening the inspiratory time, and hence elongating the exhalation time, can also aid exhalation but has only a minimal benefit after decreasing the minute ventilation, given the low flow rates observed toward the end of exhalation in patients with obstruction and the observation that decreases in inspiratory time are often limited by PVA.9 In contrast to patients on ventilation, patients with asthma rarely have difficulty with oxygenation, and so high Fio2 requirements should prompt evaluation for a superimposed problem (eg, pneumothorax, pneumonia).
Optimizing MV can be difficult in severe asthma due to a combination of severe underlying disease and often marked PVA, requiring careful medical management to achieve MV targets. As mentioned, medical management frequently involves the use of narcotic analgesics and sedatives to blunt the respiratory drive and improve the patient’s comfort in order to decrease dynamic hyperventilation. In particularly challenging cases, management may include the use of deep sedation and high-dose narcotic analgesics, neuromuscular blockade, helium-oxygen mixed gas to improve laminar flow, and, rarely, extracorporeal life support for carbon dioxide removal.9
While a detailed discussion of the medical management of status asthmaticus is beyond the scope of this article, it should be stressed that involvement of a critical care physician in the management of cases of severe asthma requiring MV should be requested early so as to prevent complications and optimize clinical outcomes.
Save Lives and Do No Harm
MV is lifesaving therapy for patients with acute respiratory failure, but it needs to be individualized for each patient’s specific underlying physiologic derangements in order to maximize its benefits and minimize its harms. Early optimization of ventilator settings should be aimed at counteracting respiratory and cardiac pathophysiology, fully supporting the work of breathing, preventing ventilator-induced lung injury, and minimizing PVA.
While they are similar in some respects, the MV management of COPD and asthma differ in the titration of PEEP, the etiology of hyperinflation, and lung compliance (Table 2). Clinicians should apply their knowledge of the unique pathophysiology of each disease to use MV as a tool both to save patients from near-death exacerbations and to spare them from the complications of MV.

Brooks T. Kuhn, MD, is an assistant professor of medicine in the Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at UC Davis Medical Center in Sacramento, California.
Jimmy Nguyen, RRT, is a registered respiratory therapist in the Department of Respiratory Care at UC Davis Medical Center in Sacramento, California.
Nicholas J. Kenyon, MD, MAS, is a professor of medicine and chief of the Division of Pulmonary, Critical Care, and Sleep Medicine at UC Davis Medical Center in Sacramento, California.
Jason Y. Adams, MD, MS, is an assistant professor of medicine in the Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at UC Davis Medical Center in Sacramento, California.
References:
- Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947-1953.
- Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17(6):648-657.
- Klein Klouwenberg PMC, van Mourik MSM, Ong DSY, et al; MARS Consortium. Electronic implementation of a novel surveillance paradigm for ventilator-associated events: feasibility and validation. Am J Respir Crit Care Med. 2014;189(8):947-955.
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126-2136.
- Chanques G, Kress JP, Pohlman A, et al. Impact of ventilator adjustment and sedation–analgesia practices on severe asynchrony in patients ventilated in assist-control mode. Crit Care Med. 2013;41(9):2177-2187.
- Reddy RM, Guntupalli KK. Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(4):441-452.
- Ward NS, Dushay KM. Clinical concise review: mechanical ventilation of patients with chronic obstructive pulmonary disease. Crit Care Med. 2008;36(5):1614-1619.
- Marini JJ. Ventilator-associated problems related to obstructive lung disease. Respir Care. 2013;58(6):938-949.
- Leatherman J. Mechanical ventilation for severe asthma. Chest. 2015;147(6):1671-1680.
- MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):530-535.
- Leatherman JW. Mechanical ventilation in obstructive lung disease. Clin Chest Med. 1996;17(3):577-590.
- Gilstrap D, MacIntyre N. Patient–ventilator interactions: implications for clinical management. Am J Respir Crit Care Med. 2013;188(9):1058-1068.
- Mellott KG, Grap MJ, Munro CL, et al. Patient ventilator asynchrony in critically ill adults: frequency and types. Heart Lung. 2014;43(3):231-243.
- Leatherman JW. Mechanical ventilation for severe asthma. Respir Care. 2007;52(11):1460-1462.
- Leatherman JW, Ravenscraft SA. Low measured auto-positive end-expiratory pressure during mechanical ventilation of patients with severe asthma: hidden auto-positive end-expiratory pressure. Crit Care Med. 1996;24(3):541-546.


