Peer Reviewed
Overactive Bladder in the Older Woman
Introduction
Overactive bladder (OAB) is the syndrome of lower urinary tract symptoms (LUTS), defined by the International Continence Society (ICS) in 2002 as “urgency, with or without urge incontinence, usually with frequency and nocturia.”1 Urgency, the sudden and compelling, often difficult (or impossible) to delay, desire to urinate is the hallmark of the OAB syndrome.2 The symptom complex of OAB represents a final common pathway of dysfunctions relating to the lower urinary tract and its neurophysiologic control, concurrent systemic morbidities and pharmacology, and environmental interactions. Thus, the term overactive bladder probably should not be used as a diagnostic term, despite its widespread implication as a diagnosis in common clinical usage. Nonetheless, the use of the term among practitioners is generally understood to refer to the presence of the symptom complex in the absence of identifiable and treatable cause.
OAB is an important health problem in older women. The overall prevalence of OAB in both men and women increases with age, with notable increases in incontinence associated with OAB in women after age 40. The National Overactive BLadder Evaluation (NOBLE) program reported a prevalence of OAB in nearly one-third of women over 65 years of age, with approximately two-thirds of these women having associated incontinence.3 Patients with OAB have significant impairments in quality of life as compared with patients who have other chronic diseases. The financial costs of OAB are considerable and have been estimated to be similar to other common problems of older women, such as osteoporosis, gynecologic and breast cancers, pneumonia and influenza, and arthritis.4
The evaluation and treatment of OAB is aimed at discovering causes that may be corrected or compensated. While the clinical syndrome of OAB is by definition the same in older women as in other groups, the factors contributing to OAB are influenced by age and gender; thus, older women represent a unique clinical subgroup of patients with OAB.
Lower Urinary Tract Function and Dysfunction in the Older Woman
The lower urinary tract consists of two functional units: the reservoir (urinary bladder) and the outlet (urethra, bladder neck, external urethral sphincter, and striated muscles of the pelvic floor). It is innervated by an integrated afferent and efferent neuronal complex involving the parasympathetic, sympathetic, and somatic neurons, regulating the muscular activities of the bladder, urethral, and periurethral musculatures.5 Lower urinary tract function (storage and emptying) is ultimately a response to bladder and urethral sensory information. The micturition reflex is mediated by pathways integrating higher cortical centers with bladder afferent and efferent stimulation. During the filling phase, bladder afferents (primarily small-diameter myelinated A delta fibers) relay sensations of bladder volume to central processing centers. Functional magnetic resonance imaging (MRI) studies demonstrate complex interactions of the periaqueductal gray (PAG), thalamus, insula, dorsal anterior cingulate gyrus (ACG), orbitofrontal cortex, and pontine micturition center (PMC).6 Under normal circumstances, these operate with subconscious control until normal volume thresholds are reached, at which time the micturition reflex may be activated at a socially appropriate moment. Efferent signaling is relayed over parasympathetic and somatic nerves, resulting in activation of the detrusor muscle via stimulation of (primarily) M3 muscarinic receptors in the detrusor muscle, and sphincteric relaxation, resulting in normal bladder emptying. Urethral afferent firing in response to flow augments and prolongs the micturition cycle.7,8 Under normal circumstances, this system allows for voluntary control over bladder function at socially acceptable voiding intervals and locations.
Since OAB is a clinical description of symptoms rather than a pathologic diagnosis, defining a precise cause of OAB is often elusive. The etiology of OAB is typically classified as neurogenic, myogenic, or autonomous, suggesting relatively isolated defects in bladder control. For example, OAB is commonly regarded as being synonymous with detrusor overactivity (ie, measurable nonvoiding contractile bladder activity or the so-called “spastic bladder”). However, fewer than half of women with urgency have demonstrable detrusor overactivity on cystometry.9 In the absence of polyuria, the final common pathway of OAB is the result of abnormal volume perceptions and/or impaired bladder volume management. Volume perceptions are affected by bladder volume, volume sensory transduction, afferent nerve integrity, central processing, and conscious perceptions. Bladder volume management, the relationship of filling volumes and ability to empty that volume, is determined by the integrity of the micturition reflex and efferent neuronal integrity, detrusor motor capabilities, bladder outlet function, sphincteric abilities, and normal sensory feedback mechanisms. Aging, comorbidities, and, to perhaps a lesser extent, gender potentially contribute to abnormalities of both perceptions and volume management abilities.
Clear definition of age-related changes in the lower urinary tract without actual disease has been hampered by the lack of definition of the normal aging bladder. Pfisterer et al10 attempted to identify age-associated changes in the female lower urinary tract in a cohort of 85 ambulatory, community-dwelling women without dementia who were assessed with bladder diaries and physiologic testing. The results revealed a decrease in bladder sensation, detrusor contractility, and urethral closure pressure associated with age, as well as an increase in nocturnal micturition. There was no reported decline in bladder capacity as the women aged, and, despite the decreased contractility, there was no significant difference in voided volume. These changes may not apply to the frail elderly population, as the oldest subgroup in the sample had a mean age of only 69 years. In elderly women with detrusor overactivity, evidence exists that there is associated decreased bladder capacity.11 This involuntary contraction in the elderly is not associated with the increased contraction strength that is seen in younger patients. This is in line with the combination of detrusor overactivity (“hyperactivity”) with impaired contractility reported as a cause of incontinence and irritative symptoms in the elderly.11 Detrusor structural and functional changes have been associated with aging.
Detrusor overactivity is associated with ultrastructural changes including increased numbers of gap junctions, which could facilitate propagation of a contraction. These changes have also been reported in aged bladders.12 Age-induced morphologic changes also include a decrease in detrusor muscle to collagen ratio and a decrease in axonal content. Elbadawi et al12 described ultrastructural changes in elderly patients with detrusor overactivity in the absence of bladder outlet obstruction. These changes included moderately widened intercellular spaces, scarce intermediate muscle cell junctions, abundant distinctive protrusion junctions, and ultra-close cell abutments.12 The latter are proposed as a possible manifestation of muscle cell dedifferentiation associated with natural aging, as well as the mediator in the propagation of involuntary contractions.12,13 Age-related increases in the release of acetylcholine directly from human urothelium and suburothelium during bladder wall stretch, as well as increases in purinergic signaling, may also play a role in the development of OAB symptoms in older persons.14
The complexity of central processing in OAB has been investigated with functional MRI. Bladder filling is associated with responses of the PAG and right insula; thus, these areas are associated with bladder volume sensing. The ACG, associated with the limbic system and PMC, are normally not activated during bladder filling. Increasing age is associated with decreased responses in the right insula, suggesting diminished responsiveness to bladder volume. In elderly women with urge incontinence, studies demonstrate strong activation of the ACG (emotional nervous center) at lower volumes as compared with younger patients with urge incontinence, potentially contributing to sensations of urgency and urge incontinence.6 Age-associated white matter hyperdensities in the brain have been implicated in affecting connections between key regions involved in micturition and thus changing control for suppression of urgency.15
Impaired voiding detrusor function and subsequent defects in bladder volume management may contribute directly and indirectly to OAB. Detrusor underactivity (DU) is an underdiagnosed condition defined by the Standardisation Sub-Committee of the ICS as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or failure to achieve complete bladder emptying within a normal time span.”1 Nearly two-thirds of institutionalized elderly persons with incontinence demonstrate evidence of DU,16 with three-fourths of symptomatic elderly women with urinary retention having urodynamic findings including DU.17 The etiology is likely a dynamic process involving structural and functional tissue changes, potentially including age-associated bladder afferent signaling dysfunction.18 DU is frequently accompanied by detrusor overactivity in the elderly population,17 a syndrome termed detrusor hyperactivity with impaired contractility (DHIC). DHIC may be associated with incontinence, urinary urgency, frequency, weak flow rate, and urinary retention. Despite described structural and neuropharmacologic changes, degradation of the detrusor motor contractile ability (ie, the ability of the detrusor muscle to generate expulsive pressure in response to normal stimulation) has not been conclusively demonstrated,19 suggesting a primacy of sensory, central, and outlet functions in the generation of OAB symptoms.
OAB in the Elderly Female
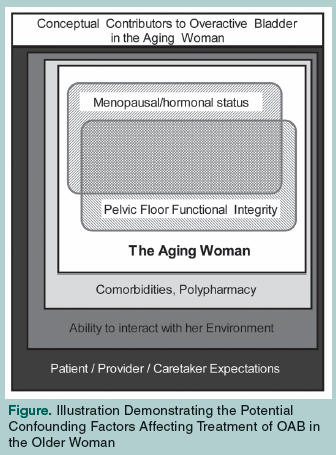 OAB in women occurs on a fundamentally different backdrop than in men. In contrast to the obstructive effect of the prostate in men, perception and control of the urinary bladder in women is uniquely affected by loss of pelvic organ support (ie, pelvic organ prolapse [POP]) and loss of estrogenization at menopause. Additionally, sphincteric incompetence—more common in women—may result in inappropriate urethral stimulation, potentially resulting in sensations of urgency, leakage, and frequency. OAB in older women represents an interplay of lower urinary tract function, aging, hormonal status, and pelvic floor function (dynamic and static pelvic organ support structures) on the background of the individual’s health status and environmental interactions. This concept is illustrated in the Figure.
OAB in women occurs on a fundamentally different backdrop than in men. In contrast to the obstructive effect of the prostate in men, perception and control of the urinary bladder in women is uniquely affected by loss of pelvic organ support (ie, pelvic organ prolapse [POP]) and loss of estrogenization at menopause. Additionally, sphincteric incompetence—more common in women—may result in inappropriate urethral stimulation, potentially resulting in sensations of urgency, leakage, and frequency. OAB in older women represents an interplay of lower urinary tract function, aging, hormonal status, and pelvic floor function (dynamic and static pelvic organ support structures) on the background of the individual’s health status and environmental interactions. This concept is illustrated in the Figure.
It is well established that the lower urinary tract is estrogen-sensitive, yet the impact of estrogen withdrawal on the development of OAB is inconclusive. A hormonal impact on lower urinary tract function is suggested by the approximately 70% of postmenopausal women with incontinence who relate the onset of their incontinence to the time of their menopause.20 Estrogen receptors are located throughout the lower urinary tract, including the musculature of the pelvic floor and the squamous epithelium of the proximal and distal urethra, the vagina, and the trigone of the bladder. Estrogen increases the cell maturation of these epithelial structures, and low estrogen may contribute to bladder muscle cell dedifferentiation and ultrastructural changes seen in impaired contractility.21 Estrogens are also known to have an effect on collagen composition in the lower urinary tract. Studies have shown that the use of exogenous estrogen therapy results in a reduction of the total collagen concentration, a decrease in the cross-linking of collagen, and an increase in levels of collagen degradation products in periurethral tissues.22,23 In animal models, estrogen has been shown to directly affect detrusor function through modifications of muscarinic receptors and by reducing the amplitude and frequency of spontaneous and rhythmic detrusor contractions.24-26 The ultimate functional effects of estrogen are likely on bladder (and perhaps urethral) sensory function27 as well as detrusor motor function.28
POP and OAB often coexist in the elderly female population; however, it is unclear if there is a casual relationship. Pelvic organ prolapsed can be defined as an abnormal loss of support of one or more of the pelvic organs, leading to prolapse into or outside of the vagina.29 Up to 50% of women who delivered vaginally have some degree of prolapse, although only 20% of these women are symptomatic.30 Prolapse symptoms are nonspecific, and the correlation between patient symptoms and clinical POP staging is known to be poor. The only symptom that has been shown to correlate with POP severity is the presence or sensation of a vaginal bulge.31 Patients may report symptoms related to sexual dysfunction (dyspareunia, obstructed intercourse, vaginal pressure), bowel function (constipation, fecal incontinence, tenesmus), lower-back pain, and LUTS (incontinence, urge, nocturia, difficulty with bladder emptying). It has been reported that 25-70% of women with POP experience OAB symptoms.31 While often attributed to “nerve stretch,” the pathophysiology is unclear. Bladder outlet obstruction may contribute, particularly if the mid-upper vagina is prolapsed in the absence of significant loss of urethral support, and should be considered prior to treatment.
Age-associated factors external to genitourinary function and its inherent regulatory pathways can contribute to or cause OAB. Diseases such as stroke can affect the central nervous system’s control of the lower urinary tract, impair mobility, and cause cognitive deficits, all of which can cause or contribute to OAB-like symptoms. Parkinson’s disease can cause impaired mobility and cognition in its late stages. Cardiovascular conditions such as congestive heart failure and venous insufficiency can contribute to urinary frequency and nocturia due to fluid mobilization, particularly during the recumbent sleeping hours. Sleep apnea can increase complaints of nocturia. Degenerative joint disease can impair mobility, and diabetes mellitus can exacerbate OAB symptoms through increased urinary tract infection, polyuria, and diabetic neuropathic bladder. Constipation and obesity have been associated with LUTS. Many older patients have poor bowel habits that result from low fiber and fluid intake. The latter may be in response to OAB symptoms. Constipation and fecal impaction may occur and may cause either irritation or obstruction in the lower urinary tract and exacerbate OAB symptoms. Treatment of multiple medical comorbidities leads to polypharmacy directly contributing to OAB as well as complicating OAB therapy. Finally, depression and dementia are associated with OAB; the pathophysiology and treatment of these disorders have direct impact on central aspects of lower urinary tract regulation. The relationship is complex, as such disorders may also contribute to loss of the ability to gage perceptions and judge social appropriateness, confounding the very meaning of “OAB.”
Evaluation of OAB in the Older Woman
Less than half of women with incontinence seek medical attention.32 Many women find discussing symptoms embarrassing and others believe it is a natural part of aging. Due to the high prevalence of OAB in elderly women, it should be included in the review of systems in all older women.
Success of treatment of OAB is linked to expectations and motivation. An important aspect of evaluation is to identify not only the symptoms, but also who is symptomatic—the patient or the caregiver. As a team, the clinician, patient, and/or caregiver should establish clear outcome goals prior to initiating evaluation. This should include improvement in specific or the most bothersome symptoms and/or lifestyle expectations. The specific nature of the evaluation will be determined by treatment goals, which are tempered by considerations of comorbidities, risks, and costs.
 The algorithm from the Fourth International Consultation on Incontinence33 defines the basic assessments needed in the evaluation of OAB in community-dwelling and frail elderly patients. For both groups, it is necessary to take a full and thorough history targeted to identify the type, severity, duration, and burden of incontinence, and any potentially modifiable contributing factors (eg, medications, comorbidities). Office-based questionnaires may be helpful; a simple three-item questionnaire and a urinalysis have been shown in a multicenter study to be safe and fairly accurate in distinguishing sphincteric (stress) incontinence from urge incontinence.34 A directed physical exam is recommended, including abdominal examination, pelvic examination (to evaluate for masses, estrogen status, POP, etc), measurement of a postvoid residual (PVR) urine volume, and a cough stress test and rectal exam to evaluate for constipation, pelvic floor muscle tone, and sphincteric competence. The variability of PVR urine volume in the frail elderly limits its utility in this population. The impact of the PVR urine volume is perhaps best understood in the context of the patient’s typical filling capacity. Urinalysis may disclose evidence of bladder irritants such as infection or tumor. A voiding diary charting 3 days of urinary habits is also recommended. In the frail elderly, evaluation of functional and cognitive assessment (including screening for depression), and assessment of patient prognosis and life expectancy should be performed. Because OAB affects quality of life, formal baseline measures of quality of life may be useful. Disease-specific questionnaires such as the International Consultation on Incontinence Modular Overactive Bladder Questionnaire are suitable for this purpose.35 Table I presents one system of evaluation incorporating these concepts into two 15-minute office visits.
The algorithm from the Fourth International Consultation on Incontinence33 defines the basic assessments needed in the evaluation of OAB in community-dwelling and frail elderly patients. For both groups, it is necessary to take a full and thorough history targeted to identify the type, severity, duration, and burden of incontinence, and any potentially modifiable contributing factors (eg, medications, comorbidities). Office-based questionnaires may be helpful; a simple three-item questionnaire and a urinalysis have been shown in a multicenter study to be safe and fairly accurate in distinguishing sphincteric (stress) incontinence from urge incontinence.34 A directed physical exam is recommended, including abdominal examination, pelvic examination (to evaluate for masses, estrogen status, POP, etc), measurement of a postvoid residual (PVR) urine volume, and a cough stress test and rectal exam to evaluate for constipation, pelvic floor muscle tone, and sphincteric competence. The variability of PVR urine volume in the frail elderly limits its utility in this population. The impact of the PVR urine volume is perhaps best understood in the context of the patient’s typical filling capacity. Urinalysis may disclose evidence of bladder irritants such as infection or tumor. A voiding diary charting 3 days of urinary habits is also recommended. In the frail elderly, evaluation of functional and cognitive assessment (including screening for depression), and assessment of patient prognosis and life expectancy should be performed. Because OAB affects quality of life, formal baseline measures of quality of life may be useful. Disease-specific questionnaires such as the International Consultation on Incontinence Modular Overactive Bladder Questionnaire are suitable for this purpose.35 Table I presents one system of evaluation incorporating these concepts into two 15-minute office visits.
Treatment Recommendations
The recent Agency for Healthcare Research and Quality evidence-based review of OAB concluded that there was no high-quality evidence to inform clinical decision-making, and so, definitive conclusions about treatment outcomes cannot be made.36 There is evidence demonstrating that both behavioral and pharmacologic treatment provide modest results for OAB symptoms. Re-evaluation of patients and therapy goals may be appropriate for those in whom standard therapies fail to address symptoms and/or therapeutic goals.
Behavioral changes are the mainstay of OAB treatment. Behavioral therapy was shown to be more effective than medications in a comparative effectiveness trial, reducing incontinence episodes by 80% versus 69% for medications.37 In addition, patient satisfaction was reported to be higher in patients undergoing behavioral therapy.38 A primary component of this treatment option is pelvic muscle exercises (Kegel exercises). This technique teaches patients how to identify and exercise pelvic floor muscles to occlude the urethra and suppress detrusor contractions. Patients are instructed in proper technique with a self-help booklet (in motivated patients) or with verbal feedback or biofeedback (more involved training). One randomized, controlled study demonstrated equal effectiveness among all three techniques, with a 50-70% reduction in incontinent episodes after 8 weeks of treatment.39 Although the biofeedback and verbal feedback interventions were not significantly more effective than the self-help intervention, they did result in better outcomes in patients’ perceptions of and satisfaction with progress. The authors postulated that this difference was possibly related to lack of contact with clinical staff.39
In patients who are cognitively intact, bladder training can be used. This includes urge-suppression techniques and other lifestyle changes such as scheduled voiding, fluid management, and reduction in caffeine intake. Patients are instructed to stand still or to sit down using relaxation techniques to curb urgency. Once this is controlled, patients slowly walk to a restroom. This is repeated until voiding intervals are 3-4 hours without leakage. This technique takes several weeks to months to master, and requires a motivated patient. For patients who are not cognitively intact, prompted or scheduled voiding is the primary element. This can be labor-intensive and difficult to sustain with patients who are institutionalized.
Postmenopausal women with symptoms of OAB are commonly treated with oral or topical estrogen. Few data document the efficacy of these agents. Cardozo et al40 reviewed 11 randomized, placebo-controlled trials involving a total of 430 women (236 women treated with estrogen and 230 women with placebo). Meta-analysis revealed statistically significant improvement in urge incontinence, frequency, and nocturia as compared with placebo. Estrogen was administered in a variety of different preparations (conjugated estrogen, estradiol, and estriol) and used either systemically or locally. The authors concluded that local administration was most effective.40 Combination therapy of estrogens and antimuscarinics may prove to provide additional benefit over either single approach.41
Antimuscarinics are the established and standard pharmacologic approach to OAB treatment. (Table II presents currently available Food and Drug Administration [FDA]–approved agents.) No one drug is demonstrably more effective for the treatment of OAB in the older woman. Studies are limited by a lack of head-to-head comparisons of all agents, and typically relatively short (12 wk) follow-up. In addition, many of the studies excluded older people by virtue of coexisting medical problems, and results should be considered in light of this fact. When prescribing these medications, it is important to note potentially additive effects of polypharmacy commonly seen in elderly patients. There is a known effect of medications with anticholinergic properties on the cognitive properties of the elderly, and special consideration should be taken when prescribing antimuscarinics to patients with cognitive impairments. There is conflicting evidence about cognitive decline and antimuscarinic use in the elderly. A recent study did not show an increase in delirium in patients who were institutionalized and taking oxybutynin 5 mg42; however, careful review of patients on antimuscarinics is advised. There is evidence that cholinesterase inhibitors used to improve cognition may precipitate incontinence. The benefits of the cholinesterase inhibitor need to be balanced with the burden of incontinence in such cases to decide on medication withdrawal or change. Women with POP and OAB symptoms that are stage II or greater should be informed of the reduced efficacy of antimuscarinics in treating their urinary symptoms.43 Surgery to correct POP is an option once standard therapies have failed.

For those patients in whom alternate therapies are desirable, neuromodulation and botulinum toxin (BoNT) injections are available. BoNT is not FDA-approved for OAB; however, it has been of interest for disorders of lower urinary tract function since Dykstra’s first report of its use in neurologic patients in 1988.44 Its use for nonneurogenic OAB was first reported within the past decade.45,46 The toxin is injected into the bladder base and trigone cystoscopically, and ostensibly acts by inhibiting acetylcholine release at the presynaptic cholinergic junction. However, there is evidence suggesting that its effect may be directly on volume sensory transduction,47,48 rather than a strict motor paralysis. The chemical denervation that results is a reversible process since axons resprout in approximately 3-6 months, requiring repeat injections every 6-9 months. The goal of treatment is to provide a dose large enough to decrease the symptoms of OAB without causing urinary retention. Increases in quality of life in up to 90% of patients with idiopathic OAB have been reported.49 There are little data specific to the use of BoNT in the elderly; a single study has reported on a cohort of 18 women and three men with a mean age of 81 years, injected for refractory OAB. Three-quarters of patients reported significant improvement, with no patients suffering from urinary retention.50 However, impaired voiding function has been reported as a complication in more than 40% of patients,51 although the clinical significance of this fact is unknown. The use of BoNT in older women should be approached with considerable caution.
Neuromodulation therapy targets nerves in the sacral plexus that control the pelvic floor and bladder function. Two approaches are currently available: posterior tibial nerve stimulation (PTNS) and sacral nerve stimulation (SNS). PTNS is performed by means of electrical stimulation of the posterior tibial nerve via a fine needle inserted percutaneously near the ankle. Patients receive treatments once weekly for 12 weeks and repeated as needed. Only one study has compared PTNS to standard antimuscarinic therapy, with findings of similar efficacy in a varied population.52 Treatment effectiveness appears to depend upon continued therapy,53 which may limit its acceptability to patients of limited mobility. There are no data regarding PTNS specific to the elderly.
SNS therapy is FDA-approved for refractory cases of urgency/frequency and urge incontinence. It involves electrical stimulation of the sacral nerves via an implanted system that includes a lead/electrode placed into the S3 foramen. Its exact mechanism of action is unclear, but it appears to be related to modulation of pelvic and pudendal afferent nerve signaling.54 Approximately half of patients who undergo preliminary testing achieve results and receive a permanent device. There are several important contraindications to SNS that impact the elderly. In patients with anatomic changes such as bony abnormalities of the sacrum, transforaminal access may be difficult or impossible. Patients with spinal stenosis should not have difficulty. Other contraindications include future need for MRI, which can render the device nonfunctional, and patients with other pacing devices such as a cardiac pacemaker because of the concern of device interference while programming. Patients with cognitive impairment are not good candidates because of the need to operate the device and provide appropriate feedback.55 Only one small study by Amundsen and Webster56 evaluated the use of SNS specifically in an older population with incontinence. Twenty-five patients over age 55 years (average age, 69 yr; range, 55-78 yr) underwent test implantation; 12 of those responded to test stimulation and were implanted. This small study demonstrated that the procedure has a low morbidity, equal to that seen in a younger population. The authors noted that while symptom improvement rates are favorable, the ability to achieve complete dryness may be less than in younger populations. Age greater than 55 years, more than three comorbidities, and neurologic disease have been associated with poorer outcomes.57
The Fourth International Consultation on Incontinence34 recommends that women with “complicated” incontinence be referred to a specialist. Those with hematuria, recurrent urinary tract infections, POP past introitus or associated with pain, symptoms after pelvic irradiation or radical pelvic surgery, or incontinence with neurologic symptoms should be referred for specialized management. Women not responding to therapy should be re-evaluated and referral/consultation should be considered.
Conclusion
OAB is a highly prevalent and important clinical problem for older women. Symptoms of urinary frequency, urgency, and incontinence should be included in the review of systems for every elderly woman. There are multiple factors that may cause or precipitate OAB symptoms, which must be addressed to ensure correct diagnosis and the best possible chance of success with treatment. Evaluation must extend beyond lower urinary tract responses to volume. Therapeutic targets including those beyond detrusor motor modulation must be considered.
The authors report no relevant financial relationships.
Dr. Harris is a Resident in Urology, and Dr. Smith is Assistant Professor of Urology and Gynecology, Department of Surgery, University of Connecticut Health Center, Farmington.
References
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-Committee of the International Continence Society. Neurourol Urodyn 2002;21(2):167-178.
2. Michel MC, Chapple CR. Basic mechanisms of urgency: Preclinical and clinical evidence. Eur Urol 2009;56(2):298-308. Published Online: May 26, 2009.
3. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20(6):327-336. Published Online: November 15, 2002.
4. Tubaro A. Defining overactive bladder: Epidemiology and burden of disease. Urology 2004;64(6 suppl 1):2-6.
5. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 2008;9(6):453-466.
6. Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage 2007;37(1):1-7. Published Online: May 18, 2007.
7. Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett 2004;360(1-2):9-12.
8. Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol 1999;517(pt 2):599-605.
9. Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? J Urol 2006;175(1):191-195.
10. Pfisterer MH, Griffiths D, Schafer W, Resnick NM. The effect of age on lower urinary tract function: A study in women. J Am Geriatr Soc 2006;54(3):405-412.
11. Pfisterer MH, Griffiths DJ, Rosenberg L, et al. The impact of detrusor overactivity on bladder function in younger and older women. J Urol 2006;175(5):1777-1783.
12. Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: Normal versus impaired contractility. J Urol 1993;150(5 pt 2):1657-1667.
13. Elbadawi A, Diokno AC, Millard RJ. The aging bladder: Morphology and urodynamics. World J Urol 1998;16 (suppl 1):S10-S34.
14. Yoshida M, Homma Y, Inadome A, et al. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol 2001;36(1):99-109.
15. Tadic SD, Griffiths D, Murrin A, et al. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage 2010;51(4):1294-1302. Published Online: March 17, 2010.
16. Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N Engl J Med 1989;320(1):1-7.
17. Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology 2007;69(3):436-440.
18. Smith PP. Aging and the underactive detrusor: A failure of activity or activation? Neurourol Urodyn 2010;29(3):408-412.
19. Michel MC, Barendrecht MM. Physiological and pathological regulation of the autonomoic control of urinary bladder contractility. Pharmacol Ther 2008;117:297-312. Published Online: December 23, 2007.
20. Iosif CS, Bekassy Z. Prevalence of genitourinary symptoms in the late menopause. Acta Obstet Gynecol Scand 1984;63:257-260.
21. Taylor JA 3rd, Kuchel GA. Detrusor underactivity: Clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc 2006;54:1920-1932.
22. Falconer C, Ekman-Ordeberg G, Blomgren B, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand 1998;77(1):95-100.
23. Keane DP, Sims TJ, Abrams P, Bailey AJ. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. BR J Obstet Gynaecol 1997;104:994-998.
24. Batra S, Andersson KE. Oestrogen-induced changes in muscarinic receptor density and contractile responses in the female rat urinary bladder. Acta Physiol Scand 1989;137:135-141.
25. Shenfield OZ, Blackmore PF, Morgan CW, et al. Rapid effects of estriol and progesterone on tone and spontaneous rhythmic contractions of the rabbit bladder. Neurourol Urodyn 1998;17:408-409.
26. Long CY, Hsu CS, Shao PL, et al. Effect of ovariectomy on the gene expression of detrusor muscarinic receptors in female rats. Fertil Steril 2009;92(3):1147-1149. Published Online: March 27, 2009.
27. deGroat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 2009;(194):91-138.
28. Valeri A, Brain KL, Young JS, et al. Effects of 17beta-oestradiol on rat detrusor smooth muscle contractility. Exp Physiol 2009;94(7):834-846. Published Online: April 24, 2009.
29. Haylen BT, deRidder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct 2010;21:5-26. Published Online: November 25, 2009.
30. Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:497-503. Published Online: June 29, 2005.
31. Burrows LJ, Meyn LA, Walters MD, Weber AM. Pelvic symptoms in women with pelvic organ prolapse. Obstet Gynecol 2004;104(5 pt 1):982-988.
32. Huang AJ, Brown JS, Kanaya AM, et al. Quality of life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med 2006;166(18):2000-2006.
33. Abrams P, Andersson KE, Birder L, et al; Members of Committees; Fourth International Consultation on Incontinence. Fourth International Consultation on Incontinence recommendations of the international scientific committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn 2010;29(1):213-240.
34. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med 2006;144(10):715-723.
35. Abrams P, Avery K, Gardener N, Donovan J; ICIQ Advisory Board. The International Consultation on Incontinence Modular Questionnaire: www.iciq.net. J Urol 2006;175(3 pt 1):1063-1066.
36. Vanderbilt Evidence-Based Practice Center. Treatment of overactive bladder in women. Agency for Healthcare Research and Quality. August 2009. http://www.ahrq.gov/downloads/pub/evidence/pdf/bladder/bladder.pdf. Accessed September 10, 2010.
37. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: A randomized controlled trial. JAMA 1998;280(23):1995-2000.
38. Burgio KL, Locher JL, Goode PS, et al. Psychological improvements associated with behavioral and drug treatment of urge incontinence in older women. J Gerontol B Psychol Sci Soc Sci 2001;56(1):P46-P51.
39. Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: A randomized controlled trial. JAMA 2002;288(18):2293-2299.
40. Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta Obstet Gynecol Scand 2004;83(10):892-897.
41. Tseng LH, Wang AC, Change YL, et al. Randomized comparison of tolterodine with vaginal estrogen cream versus tolterodine alone for the treatment of postmenopausal women with overactive bladder syndrome. Neurourol Urodyn 2009;28(1):47-51.
42. Lackner TE, Wyman JF, McCarthy TC, et al. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc 2008;56(5):862-870. Published Online: April 9, 2008.
43. Salvatore S, Serati M, Ghezzi F, et al. Efficacy of tolterodine in women with detrusor overactivity and anterior vaginal wall prolapse: Is it the same? BJOG 2007;114(11):1436-1438. Published Online: September 17, 2007.
44. Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139(5):919-922.
45. Dykstra D, Enriquez A, Valley M. Treatment of overactive bladder with botulinum toxin type B: A pilot study. Int Urogynecol J Pelvic Floor Dysfunct 2003;14(6):424-426. Published Online: November 25, 2003.
46. Rapp DE, Lucioni A, Katz EE, et al. Use of botulinum-A toxin for the treatment of refractory overactive bladder symptoms: An initial experience. Urology 2004;63(6):1071-1075.
47. Vemulakonda VM, Somogyi GT, Kiss S, et al. Inhibitory effect of intravesically applied botulinum toxin A in chronic bladder inflammation. J Urol 2005;173(2):621-624.
48. Hanna-Mitchell AT, Birder LA. New insights into the pharmacology of the bladder. Curr Opin Urol 2008;18(4):347-352.
49. Schmid DM, Sauermann P, Werner M, et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholinergics. J Urol 2006;176(1):177-185.
50. White WM, Pickens RB, Doggweiler R, Klein FA. Short-term efficacy of botulinum toxin A for refractory overactive bladder in the elderly population. J Urol 2008;180(6):2522-2526. Published Online: October 19, 2008.
51. Brubaker L, Richter HE, Visco A, et al; Pelvic Floor Network Disorders. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008;180(1):217-222. Published Online: May 21, 2008.
52. Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol 2009;182(3):1055-1061. Published Online: July 18, 2009.
53. van der Pal F, van Balken MR, Heesakkers JP, et al. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: Is maintenance treatment necessary? BJU Int 2006;97(3):547-550.
54. Leng WW, Chancellow MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am 2005;32:11-18.
55. McAchran S, Daneshgari F. Sacral neuromodulation in the older woman. Clin Obstet Gynecol 2007;50:735-744.
56. Amundsen CL, Webster GD. Sacral neuromodulation in an older, urge-incontinent population. Am J Obstet Gynecol 2002;187(6):1462-1465.
57. Amundsen CL, Romero AA, Jamison MG, Webster GD. Sacral neuromodulation for intractable urge incontinence: Are there factors associated with cure? Urology 2005;66:746-750.


