Osteoporosis in Older Women
Introduction
Osteoporosis is a bone disease manifested clinically by fragility fractures.1 In 2005-2006 in the United States, 40 million people age 50 years and over had low bone mineral density (BMD) at the femoral neck,2 and, on average, one in every two Caucasian women will sustain an osteoporotic fracture during their lifetime.3 With the projected 60% increase in people age 50 and over by 2025, the cost of osteoporotic fractures is expected to reach $25 billion.4
Fractures are associated with significant loss of quality of life and increased morbidity, with 20% entering a nursing home following a hip fracture and 40% experiencing new functional impairment.3 Excess mortality after hip and vertebral fracture depends largely on age.5 During the first 3 months post-hip fracture, there is a fivefold increased hazard of all-cause mortality for women, and this increase in mortality declines over 10 years; however, it does not return to baseline.6
Despite these devastating consequences, osteoporosis is underdiagnosed and treatment is underutilized.7,8 This review will discuss the evaluation of osteoporosis and fracture risk in older community-dwelling postmenopausal women, treatment thresholds, and available therapies. Particular challenges in treating older women with osteoporosis will be addressed, including efficacy, adverse drug effects, cost concerns, and other factors that affect outcome.
Risk Factors
Primary osteoporosis (or age-related osteoporosis) in women results from a high bone turnover state associated with estrogen deficiency.3 The incidence of fractures increases exponentially with age, and a previous fracture increases the risk of a subsequent fracture by almost twofold.9 Approximately 20-30% of women with osteoporosis have a secondary cause for their low bone density.10
Common risk factors for osteoporosis and fractures in older women include1,3,10:
• Lifestyle: Low body weight, low calcium intake, vitamin D insufficiency, excessive alcohol intake, smoking, decreased physical activity, and falls
• Medications: Glucocorticoids, aromatase inhibitors, and anticonvulsants
• Genetic factors: Parental history of hip fracture, and osteogenesis imperfecta
• Endocrinopathies: Hyperthyroidism, hyperparathyroidism, and Cushing’s syndrome
• Gastrointestinal diseases: Celiac disease, gastric bypass, and malabsorption
• Comorbid illnesses: Rheumatic diseases, stroke, Parkinson’s disease, renal disease, multiple myeloma, and depression
Diagnosis
Dual energy x-ray absorptiometry (DXA) scan has become the gold standard for the noninvasive measurement of BMD.1 It is accurate, precise, painless, and rapid, and with little radiation exposure. The World Health Organization (WHO) defines osteoporosis as a T-score less than -2.5 standard deviations (SD) below the normal mean reference range for a young population that has reached peak bone mass.11 Osteopenia is defined as a T-score between -1.0 and -2.5 SD.
The National Osteoporosis Foundation (NOF) recommends BMD testing for the following12:
• Women age 65 and older, regardless of clinical risk factors
• Younger postmenopausal women about whom you have concern based on their clinical risk factor profile
• Women in the menopausal transition if there is a specific risk factor associated with increased fracture risk, such as low body weight, prior low-trauma fracture, or high-risk medication
• Adults who have a fracture after age 50
• Adults with a condition (eg, rheumatoid arthritis) or who are taking a medication (eg, glucocorticoids in a daily dose ≥ 5 mg prednisone or equivalent for ≥ 3 mo) associated with low bone mass or bone loss
• Anyone being considered for pharmacologic therapy for osteoporosis
• Anyone being treated for osteoporosis, to monitor treatment effect
• Anyone not receiving therapy in whom evidence of bone loss would lead to treatment
For patients or clinical settings in which it is not practical to obtain DXA, the WHO Fracture Risk Assessment Tool, or FRAX®, was developed to calculate a 10-year fracture risk based on clinical risk factors (www.shef.ac.uk/FRAX). FRAX can also be used in conjunction with BMD testing to enhance the specificity for fracture prediction. The model was constructed from the primary data of nine international population-based cohorts and was validated in 11 independent cohorts (mainly women).13
A limitation of this tool is its failure to incorporate a number of risk factors for fracture in older adults, including falls risk, number of previous fractures, dose and duration of exposure to bone-active substances, and common comorbidities that increase fracture risk such as Parkinson’s disease and stroke. Furthermore, because age is such a powerful risk factor for fracture, a large proportion of women in their 80s and 90s will have a high 10-year fracture risk if FRAX is used without concomitant BMD testing, thus limiting its usefulness. FRAX may be helpful for older women with osteopenia when the decision to treat is unclear,14 although it should be emphasized that this approach has not been tested in randomized, controlled trials.
Treatment Considerations
The NOF suggests that postmenopausal women age 50 and older presenting with the following should be considered for treatment12:
• A hip or vertebral (clinical or morphometric) fracture regardless of BMD
• T-score ≤ -2.5 at the femoral neck or spine after appropriate evaluation to exclude secondary causes
• Low bone mass (T-score between -1.0 and -2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture ≥ 3% or a 10-year probability of a major osteoporosis-related fracture ≥ 20% based on FRAX
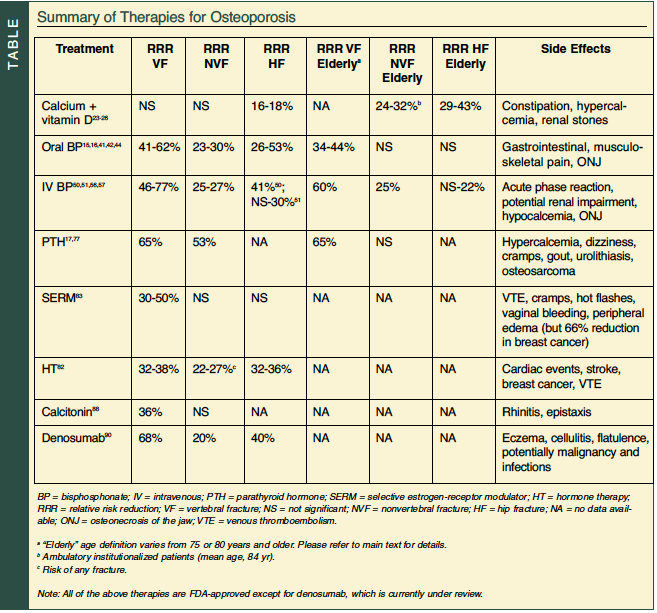
Many efficacious interventions are available for the prevention and treatment of osteoporosis (Table); however, treating older patients poses a significant challenge. Declining renal function, chronic illnesses, and polypharmacy potentially increase adverse events; cognitive impairment, cost concerns, and many other factors affect compliance and outcome. Discontinuation rates of oral bisphosphonates can be as high as 11-45%.15
Many trials have excluded older patients from participation, and so there is a paucity of data regarding treatment efficacy in the oldest old. However, available evidence suggests that pharmacologic therapy remains safe in all age subgroups and in nursing home residents,16-18 and that because of the higher absolute risk of fractures, older subjects may actually achieve a greater absolute risk reduction with pharmacologic therapy.5 Therefore, treatment should be considered even for frail older patients at a high risk of fractures whenever it is consistent with their goals of care.
Nonpharmacological Interventions
Lifestyle intervention is important, not only for bone density but for overall general health. Smoking cessation, moderation of alcohol intake, and adequate nutrition should be addressed. Weight-bearing and balance exercises may modestly increase bone density12 and should be encouraged for their overall health benefits. Hip protectors are likely to be ineffective in community-dwelling persons, and their efficacy remains controversial in nursing home residents.19,20
Falls Prevention
About 30% of community-dwelling patients age 65 and over fall each year21 and approximately 1 in 10 falls results in a fracture. While a full description of interventions for preventing falls is beyond the scope of this article, a recent review of 111 randomized trials involving 55,303 patients found that multifactorial interventions are effective in reducing the rate, but not risk, of falling.21 Exercise programs, rationalization of medication regimens, and tackling particular health problems such as cataract surgery for poor vision and cardiac pacing for carotid sinus hypersensitivity are all effective interventions.
Vitamin D and Calcium
Vitamin D possesses important skeletal and nonskeletal effects that are important in both falls and fracture prevention. It is estimated that approximately 1 billion people worldwide are vitamin D–insufficient or –deficient,22 and this has widespread health ramifications.
A recent meta-analysis found that hip fracture reduction (18% as compared to placebo) occurs only with the addition of calcium to vitamin D.23 This is supported by an updated Cochrane report, summarizing 45 trials involving 84,585 patients.24 Supplementation is also very effective in ambulatory institutionalized patients.25,26 Compliance is crucial. In the Women’s Health Initiative (WHI) study, adherence with calcium and vitamin D (1000 mg and 400 IU, respectively, for 80% of the time) was associated with a relative risk reduction (RRR) of hip fracture of 29% as compared to 12% in the intention-to-treat analysis, reinforcing the importance of compliance.27
It is important to note that clinical trials of osteoporosis therapies included calcium and vitamin D supplementation in the active treatment group, and thus any osteoporosis prescription should include a therapeutic dose of calcium and vitamin D. The prevalence of vitamin D insufficiency (25-OH vitamin D < 30 ng/mL) and deficiency (25-OH vitamin D < 20 ng/mL) among postmenopausal women who are taking anti-osteoporosis treatment in North America is high (52% and 18%, respectively).28 Furthermore, a number of studies have suggested that vitamin D insufficiency reduces the recovery of BMD in patients taking bisphosphonate therapy.29,30
The recommended daily intake of vitamin D is 800-1000 IU.12 There is a large therapeutic window for 25-OH vitamin D preparations, and toxicity is rarely an issue. The therapeutic goal for the serum 25-OH vitamin D level is 30 ng/mL.12 Women age 50 and over should have 1200 mg of elemental calcium daily. A study claimed an increase in cardiovascular and stroke events in postmenopausal women taking calcium31; however, the data are inconclusive and controversial.32-35 However, based on this preliminary data, it is reasonable to recommend that patients get 1200-1500 mg each day from diet and calcium supplements combined.
Pharmacological Therapy
Many treatment options are currently available, and a number of novel therapies are under investigation. Head-to-head trials to demonstrate superiority between agents are uncommon, and direct comparison is difficult.
Bisphosphonates
Bisphosphonates resemble pyrophosphate and are incorporated into bone to inhibit osteoclast activity and reduce bone resorption. These agents are often considered the first-line treatment of osteoporosis in older women.
Oral bisphosphonates. Alendronate, risedronate, and ibandronate are approved for the treatment of postmenopausal osteoporosis. These agents reduce the risk of vertebral fractures by 41-62% (alendronate 44-48%, risedronate 41-49%, ibandronate 62%).15 Recent meta-analyses have provided support for a beneficial effect on nonvertebral fractures.36-42 Alendronate and risedronate reduce nonvertebral (RRR 23-30%) and hip (RRR 26-53%) fractures.41,42 Ibandronate did not reduce the incidence of nonvertebral fractures in a large trial; however, a post-hoc analysis reported a significant reduction (RRR 69%) in higher-risk patients (femoral neck T-score < -3.0 SD).43
Bisphosphonates are effective and safe in older women. In a subgroup analysis of women between 75-82 years of age from the Fracture Intervention Trial (FIT), alendronate remained effective for vertebral fracture reduction (RRR 34%).44 There are no published data on nonvertebral fracture reduction for patients over age 75 for alendronate therapy.45 All studies of ibandronate excluded women over age 80 years.
The Hip Intervention Program (HIP) trial included a subset of older women (age ≥ 80 yr) selected on risk factors for hip fracture and not BMD.46 Risedronate (> 3 yr) did not reduce the risk of hip fractures in these women, and this may be due to other competing nonskeletal factors that are not amenable to pharmacologic intervention and that offset the benefit of drug intervention. A post-hoc analysis of three risedronate trials examined its efficacy in women age 80 and over.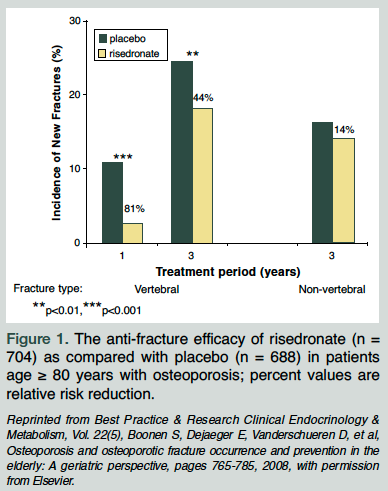 After 3 years, there was a significant reduction in new vertebral fractures versus control (RRR 44%) (Figure 15). There was no reduction in nonvertebral fractures in the older age group, and this may due to the lack of statistical power to detect a difference. The adverse event profile was similar to the control group, even in patients who had active gastrointestinal disease at baseline. In summary, risedronate is well tolerated and remains efficacious in vertebral fracture reduction in the oldest old.
After 3 years, there was a significant reduction in new vertebral fractures versus control (RRR 44%) (Figure 15). There was no reduction in nonvertebral fractures in the older age group, and this may due to the lack of statistical power to detect a difference. The adverse event profile was similar to the control group, even in patients who had active gastrointestinal disease at baseline. In summary, risedronate is well tolerated and remains efficacious in vertebral fracture reduction in the oldest old.
Extension trials provided support for the safety of bisphosphonates. The Fracture Intervention Trial Long-term Extension (FLEX) randomized women to continue alendronate or switch to placebo after 5 years of alendronate therapy.47 Alendronate maintained its effect on BMD after 10 years, and it was well tolerated. The trial was not powered to examine fracture endpoints; however, women taking placebo after 5 years of alendronate maintained BMD above their baseline level. Therefore, for some women, 5 years of bisphosphonate therapy may be enough to realize fracture reduction benefits.48 Extension trials to 7 years in total of risedronate therapy demonstrated continued anti-fracture efficacy.49
Intravenous bisphosphonates. Intravenous ibandronate and zoledronic acid (ZOL) are also effective treatment options. The Health Outcomes and Reduced Incidence with Zoledronic acid ONce yearly (HORIZON)-Pivotal Fracture Trial (PFT) randomized women (mean age, 73 yr) with and without previous vertebral fractures. Yearly ZOL showed significant reductions in morphometric vertebral (RRR 70%), hip (RRR 41%), and nonvertebral fractures (RRR 25%) over 3 years.50 HORIZON-Recurrent Fracture Trial (RFT) enrolled women and men (mean age, 74 yr) following surgical repair of a hip fracture, and median follow-up was 1.9 years.51 The RRR with ZOL for any new clinical fracture, new clinical vertebral fracture, and new nonvertebral fracture was 35%, 46%, and 27%, respectively. The rates of second hip fracture reduction were not significant. Intravenous ibandronate demonstrated noninferiority to oral ibandronate,52,53 and two post-hoc analyses confer nonvertebral fracture efficacy when higher doses of ibandronate (150 mg po monthly or 3 mg IV quarterly as compared to 2.5 mg po daily) were used.54,55
A subgroup analysis of older patients (age ≥ 75 yr) from HORIZON-PFT was performed.56,57 Similar reductions in all clinical fractures were seen for patients age 75 and older (RRR 34%) as compared to those younger than age 70 years (RRR 37%). The reduction in vertebral fractures was greater in the younger population (RRR 80% in those < 70 yr vs 60% in those ≥ 75 yr), but there were no significant treatment-by-age group interactions for hip and nonvertebral fractures. HORIZON-PFT is the first study to show a significant reduction in nonvertebral fractures with bisphosphonates among those over age 75 (RRR 25%).57
ZOL is the first anti-fracture medication to demonstrate a survival benefit.51 A 28% reduction in all-cause mortality was found (9.6% in the ZOL group and 13.3% in the placebo group). In a subsequent exploratory analysis, the adjusted reduction in mortality was 25%, and subsequent fractures explained only 8% of the mortality benefit.58 Patients were less likely to die from pneumonia and arrhythmias; however, the mechanism for the mortality effect remains unknown.
Safety of bisphosphonates. Bisphosphonates are generally well tolerated. Although the side-effect profile is well known, several safety concerns that have recently emerged will be discussed here.
There has been growing concern about oversuppression of bone remodeling and the potential risk of increased skeletal fragility. A number of case reports and case series have described unusual fracture sites, in particular the femoral diaphysis (subtrochanteric fractures), associated with alendronate intake.59-65 It is thought that when remodeling is suppressed, microcracks and microdamage go unrepaired, leading to an increased susceptibility to fractures.66 Patients may have prodromal symptoms of pain and common radiological features including thickened cortices and lateral femoral “beaking” at the fracture site. Bone biopsy results are often but not always consistent with low bone turnover and the absence of other causes of fragility. These reports do not establish a causal association between alendronate and atypical fractures, nor is it conclusive that the problem is limited to alendronate only. An increased awareness of this potential complication is warranted, and the duration of therapy should be reviewed.
There have been about 23 cases of esophageal cancer reported to the Food and Drug Administration (FDA) in those taking oral bisphosphonate therapy.67 The median time from exposure to diagnosis in these cases was 1.3-2.1 years, likely incompatible with a cause-and-effect relationship,68 and several other studies have not supported this risk.69,70
Bisphosphonates are excreted by the kidneys, and there is a lack of safety and efficacy data for use in those with a glomerular filtration rate (GFR) of less than 30 ml/min. This becomes particularly concerning in older patients with age-related reductions in creatinine clearance.71 Two post-hoc analyses of alendronate and risedronate have shown that there is no effect on renal function in patients with a GFR as low as 15 ml/min after 2-3 years of treatment.72 In HORIZON-PFT there was an increase in the creatinine (> 0.5 mg/dL), but this did return to baseline.50 Despite these reassurances, prospective studies are required, and the FDA warns against using bisphosphonates in patients with a GFR of less than 30-35 ml/min.73 When treating older patients with bisphosphonate therapy, particularly intravenous formulations, one should be mindful of the current renal function, concomitant nephrotoxic drugs, and hydration status.
After an unexpected increase in serious atrial fibrillation (AF) events in the HORIZON-PFT trial of ZOL, concern arose that bisphosphonates might increase the risk of AF.50,74 Subsequent trials and database analyses have shown inconsistent findings.51,75 While still controversial, current consensus is that any increased risk if real is small and outweighed by the substantial reduction in clinical fractures.71
Osteonecrosis of the jaw (ONJ) has been reported in patients treated with bisphosphonates, particularly higher-dose intravenous formulations used in patients with cancer. The exact pathogenesis is poorly understood, but the risk of ONJ in patients with osteoporosis seems to be very rare, with 1 case in 10,000-100,000 patients.76 A dental examination should be considered prior to treatment with intravenous bisphosphonates in patients with a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, preexisting dental disease or infection, anemia, coagulopathy).73 Because of the long half-life of bisphosphonates in bone, stopping therapy prior to dental procedures is not likely to be useful.
Parathyroid Hormone – Teriparatide
Teriparatide comprises the first 34 amino acids of the parathyroid hormone (PTH) and is an anabolic agent for bone. The Fracture Prevention Trial (FPT) randomized 1637 postmenopausal women with a previous vertebral fracture to either 20 mcg or 40 mcg of teriparatide daily, or placebo for 18 months.77 The risk of new vertebral fractures was reduced by 65% (20 mcg) and 69% (40 mcg) as compared to placebo. The risk was also reduced for new nonvertebral fragility fractures (RRR 53% and 54% for 20 mcg and 40 mcg, respectively).
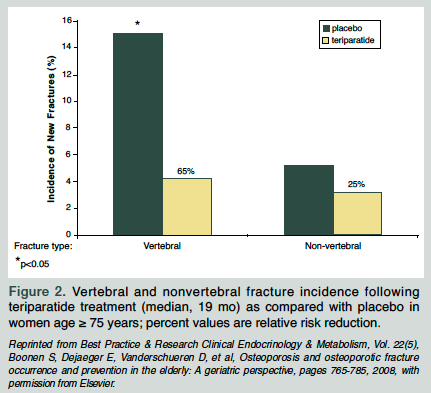 A subgroup analysis of the FPT compared treatment efficacy and safety in patients younger than 75 years with patients age 75 years and older.17 The results indicated that teriparatide was similarly effective and well tolerated in the older age group. The nonvertebral fracture reduction was similar but not significant (Figure 25).
A subgroup analysis of the FPT compared treatment efficacy and safety in patients younger than 75 years with patients age 75 years and older.17 The results indicated that teriparatide was similarly effective and well tolerated in the older age group. The nonvertebral fracture reduction was similar but not significant (Figure 25).
Combination therapy with teriparatide and alendronate showed no evidence of synergy when BMD outcomes were compared.78 Furthermore, sequential therapy (teriparatide alone for 1 yr followed by alendronate alone for 1 yr) maintained gains in BMD as compared to the loss of BMD if teriparatide was followed by no treatment.79
Continuing therapy for more than 2 years is not recommended due to the potential risk of osteosarcoma, which has been reported in rats with higher doses of teriparatide.77 No definite cases of osteosarcoma caused by teriparatide have been described in humans. It should not be prescribed for patients at increased baseline risk for osteosarcoma (eg, those with Paget’s disease of bone or unexplained elevations of alkaline phosphatase, or prior radiation therapy involving the skeleton).73
Hormone Therapy
The WHI dramatically shifted the hormone replacement prescribing practice of many physicians. Between 1993 and 1998, 161,809 postmenopausal women age 50-79 years were enrolled into a set of clinical trials.80 The details of these studies are well known, and the following will focus on fracture data.
Combined estrogen-progestin therapy was associated with lower hip fracture rates (10 per 10,000 person-years vs 15 per 10,000 person-years in the placebo group; hazard ratio [HR] 0.66).80 In the estrogen-only arm of the WHI, there were six fewer cases of hip fractures per 10,000 person-years.81 There were also reductions in clinical vertebral fractures and total fractures in both arms. It should be noted that women with severe osteoporosis were not included in the WHI.82
Because of the increased risk of breast cancer and cardiovascular events seen in the hormone therapy (HT) group, HT should only be considered for the prevention of fractures if other therapies are considered unsuitable and the risk profile for adverse events with HT is low. Older patients generally have more chronic illness and many risk factors that preclude them from HT.12,73
Raloxifene
Raloxifene is a selective estrogen-receptor modulator (SERM) and antiresorptive agent. The Multiple Outcomes of Raloxifene Evaluation (MORE) trial83 enrolled women age 31-80 years, and demonstrated that 60 mg daily reduced new vertebral fractures for those without preexisting vertebral fractures by 50%, and reduced additional vertebral fractures for those with preexisting vertebral fractures by 30% over 3 years. There was no significant reduction in nonvertebral fractures.
The Continuing Outcomes Relevant to Evista (CORE) study84 reported that women treated for 8 years with raloxifene had a 66% reduction in the risk of invasive breast cancer as compared with placebo. Furthermore, the Raloxifene Use for The Heart (RUTH) study85 randomized postmenopausal women with risk factors for or who had coronary artery disease to raloxifene (60 mg daily) or placebo for approximately 5 years. Investigators found no increased risk of coronary events, stroke, or total death; however, there was a significant increase in the risk of fatal stroke (HR 1.49). Due to the lack of data on patients over 80 years old, the potential adverse-event profile, and lack of nonvertebral fracture efficacy, the use of SERMs is limited in the older population. There are newer SERMs under investigation that appear to have nonvertebral fracture efficacy, although the risk-benefit ratio for these agents remains unclear.86,87
Calcitonin
Calcitonin is produced by the parafollicular thyroid cells and inhibits the production and activity of osteoclasts. The largest study, Prevent Recurrence Of Osteoporotic Fractures (PROOF),88 was a 5-year trial enrolling 1255 postmenopausal women with established osteoporosis. This demonstrated a 36% risk reduction in vertebral fractures and no significant reduction of nonvertebral fractures (200 IU intranasal calcitonin). Limitations to this study include partial blinding, low retention rate (59% withdrew prematurely), and the intention-to-treat analysis did not include information on over 50% of participants who were originally enrolled.89 Given the small and questionable efficacy of calcitonin and its expense, it is usually reserved for those patients who cannot tolerate other more effective agents.
Denosumab
Denosumab is a novel therapy for the treatment of osteoporosis that is currently under review by the FDA. It is a fully human monoclonal antibody directed at receptor activator of nuclear factor-kappaB ligand (RANKL) that binds to it with high affinity and inhibits osteoclastogenesis; thus, it represents a new therapeutic class.
The Fracture REduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial90 randomized 7808 women (age 60-90 yr) with osteoporosis to denosumab (60 mg subcutaneously every 6 mo) or placebo for 3 years. Significant risk reduction was shown for vertebral (68%), hip (40%), and nonvertebral (20%) fractures. There were no serious adverse effects.
The RANKL/OPG/RANK pathway is involved in the development of the immune system, and so there is the potential for increased infection and malignancy rates. Long-term safety information is therefore needed. There is a paucity of data on the safety and efficacy of denosumab in older populations or those with multiple comorbidities. However, this class of agents has the potential to add another fracture reduction option to the arsenal of osteoporosis therapies, and new data are emerging rapidly.
Economic Analyses
With escalating healthcare costs, patient management should also incorporate fiscally responsible decision-making. There are more than 40 internationally published cost-effective studies associated with osteoporosis.
A comprehensive economic analysis was published in 2005 from the United Kingdom.40 This study examined three bisphosphonates (risedronate, alendronate, etidronate), raloxifene, and teriparatide. Due to the rapidly increasing risk of fracture with age, the cost per quality adjusted life-year (QALY) decreased with increasing age, indicating that it is more economically attractive to treat older as compared to younger individuals.
A recent economic analysis examined absolute fracture risk to define intervention thresholds.91 The models were based on 5-year treatment with a bisphosphonate with a 35% anti-fracture efficacy. Treatment relative to no intervention among average-risk populations yielded incremental cost-effective ratios that ranged from over $380,000 per QALY gained in 50-year-old white women, to being cost-saving in 75-year-old white women. From this report, the NOF defined a cost-effective intervention threshold to be when the 10-year absolute risk of hip fracture is 3% in women.
A cost-effectiveness study of alendronate and teriparatide in the United States reported that 5 years of alendronate was cost-effective ($11,600 per QALY) as compared to sequential therapy (PTH 2 yr followed by alendronate 5 yr) of $156,500 per QALY.92 Teriparatide alone (2 yr) was $172,300 per QALY as compared to usual care.
Conclusion
Many exciting advances have been made in reducing the burden of osteoporosis in older patients. Enhanced tools are available to assess fracture risk, new therapeutic agents continue to broaden our armamentarium, and mortality benefits derived independent of subsequent fracture reduction all carry the momentum of positive change. The future holds great promise for achieving significant reductions in osteoporosis-related disease and economic burden.
Dr. Pham has received an educational grant from Novartis pharmaceuticals; Dr. Colón-Emeric receives research support from Wyeth and is a consultant for Novartis pharmaceuticals; and Dr. Weber receives speaker honoraria from Novartis, Roche, Eli Lilly, and Merck, and has received research grants from Proctor & Gamble and Amgen.
Dr. Pham and Dr. Weber are from the Department of Medicine, Division of Endocrinology, Duke University Medical Center, Durham, NC; and Dr. Colón-Emeric is from the Department of Medicine, Division of Geriatrics, Duke University Medical Center and the Durham VA Geriatric Research, Education and Clinical Center.


