Peer Reviewed
Nutritional Assessment of the Geriatric Patient: A Comprehensive Approach Toward Evaluating and Managing Nutrition
This is the fourth and final article in a series on nutrition in the elderly. The other articles in the series, “Vitamin D and Calcium: Implications for Healthy Aging,” “Vitamin B12: Considerations for Maintaining Optimum Health in Elders,” and “Popular Diets: Examining Weight Loss Diets for Geriatric Patients” were published in previous issues of Clinical Geriatrics®
AUTHORS:
Ellen Loreck, MS, RD, LDN; Roja Chimakurthi, MBBS; and Nanette I. Steinle, MD, RD
Series Editor: Nanette I. Steinle, MD, RD
CITATION:
Loreck E, Chimakurthi R, Steinle NI. Nutritional assessment of the geriatric patient: a comprehensive approach toward evaluating and managing nutrition. Clin Geriatr. 2012;20(4):20-26.
Nutritional issues are commonly encountered in older adults. The prevalence of malnutrition is estimated to be 23% in this population, and another 46% are considered to be at risk of malnutrition.1 Undernutrition and overnutrition, both considered forms of malnutrition, are areas of concern. It is estimated that between 30% and 60% of older adults are undernourished, with energy intakes less than two-thirds of the recommended dietary allowance (RDA),2 yet the prevalence of obesity is also increasing at an alarming rate in older adults. In 2000, 22.9% of adults aged 60 to 69 years and 15.5% of adults aged 70 years and older were considered obese,3 whereas the prevalence of obesity has more recently been estimated to be approximately 60% in individuals 65 years and older.4 There is significant variability in the rates of malnutrition, however, depending on the setting, with the highest rates of obesity observed among community-dwelling elders and the highest rates of undernutrition observed among hospitalized elders and those in acute rehabilitation settings. Although nutritional issues are prevalent in older adults, they are often underdiagnosed; thus, nutritional assessments should be a routine component of ongoing geriatric care. In this article, we discuss the definitions of malnutrition, including undernutrition and overnutrition; factors that contribute to nutritional deficiencies; tools used to assess nutritional risk; and tools used to conduct nutritional assessments, including anthropometric and body composition measurements, laboratory tests, and clinical assessments. We also discuss how to evaluate a patient’s diet history, methods for incorporating nutrient-rich foods into the nutrition plan of poorly nourished patients, and when to refer patients to healthcare professionals who can assist in addressing concerns impacting nutritional status.
Understanding Malnutrition
Malnutrition is defined as a state in which a deficiency, excess, or imbalance of energy, protein, or other nutrients causes adverse effects on body form, function, and clinical outcomes.5 Two major markers of malnutrition in older people are sarcopenia and cachexia.6 Sarcopenia is defined as a syndrome of progressive and generalized loss of skeletal muscle mass and strength, which increases the risk of adverse outcomes, such as physical disability, poor quality of life, and even death.7 Diagnosis is made based on findings of decreased muscle mass and either decreased muscle strength or decreased physical performance. Cachexia is defined as complex metabolic processes associated with an underlying illness (eg, cancer, end-stage renal disease, congestive heart failure) and is characterized by loss of muscle mass with or without loss of fat mass.7 In elders, cachexia is generally characterized by severe wasting, and it is frequently associated with inflammation, insulin resistance, and breakdown of muscle protein. Most elders with cachexia also have sarcopenia, but those with sarcopenia frequently do not have cachexia.
The terms undernutrition and malnutrition are often used synonymously, but undernutrition is actually a form of malnutrition. Undernutrition is defined as inadequate nutrition resulting from lack of food or failure of the body to properly absorb or assimilate nutrients.2 It is often characterized by a low body weight (≤100 lb) and low body mass index (BMI; ≤18.5 kg/m2), although individuals who consistently have a caloric or nutrient intake of less than two-thirds of the RDA may also be considered undernourished.2 In contrast, overnutrition is defined as a condition of excess nutrient and energy intake over time and is regarded as a form of malnutrition when it leads to obesity, which can lead to a decline in physical function and exacerbated frailty.8,9
Factors Contributing to Nutritional Deficits
Many factors contribute to malnutrition and undernutrition in elders, including social, psychological, and biological issues (Table 1). In a study of community-dwelling elders aged 75 to 85 years in New Zealand, those at higher risk of nutritional deficits tended to be spouseless or lived alone.10 De Castro11 has shown that meals eaten with others tend to be 44% larger than meals eaten alone. Arranging for individuals who have lost a spouse or those who live alone to eat with others may be a good strategy to improve the overall nutritional status in these individuals. Depression is a major contributor to malnutrition12; thus, use of a geriatric depression screening tool should be included as part of a comprehensive nutritional risk assessment.13

Physical activity levels have also been correlated with nutritional status in elders. With aging, body fat increases and muscle mass decreases, accounting for a loss of up to 3 kg of lean body mass per decade after the age of 50 years.14 The causes of increased body fat are multifactorial and include a lower resting metabolic rate, decreased physical activity levels, and decreased secretion of growth and sex hormones. Aging also causes an increase in abdominal fat, a condition associated with increased insulin resistance and higher risk of ischemic heart disease, diabetes, and stroke.15 Physical activity is an effective strategy to counter these body composition changes. Elders at lower risk of nutritional deficits are more likely to have greater muscle mass and strength and lower body fat levels, and are engaged in more physical activities.10 Individuals who live with someone else may have higher levels of physical
activity than those who live alone.16
Another important factor that may impact nutritional status is anorexia of aging. With increasing age, appetite and food intake decline. Aging is associated with dry mouth, diminished sense of taste and smell, loss of ability to chew food, and poor dentition, all of which can lead to decreased food intake. A study published by the Academy of Nutrition and Dietetics (AND) that compared differences in calorie consumption between individuals aged 25 and 70 years revealed that daily caloric intake in older men dropped by 1000 to 1200 kcal and older women took in 600 to 800 kcal less per day.17 Most likely, decreased food intake is a physiologic response to decreased energy expenditure. If the decrease in energy intake is greater than the decrease in energy output, body weight will decline. In an observational study of food choice in homebound older adults, the key factor to promoting increased food intake was incorporating foods that were tasty, convenient, and affordable.18 Being unable to shop was identified as a major barrier to eating desired foods, and participants also viewed their health and need for a special diet as interfering with what they really wanted to eat. The concept of liberalizing diets is supported by a 2010 position paper by the AND that promotes individualization to less-restrictive diets for elders living in healthcare communities.17
Nutritional Assessments
Nutritional risk screening should be integrated into the comprehensive geriatric assessment.19 Numerous screening methods are available, including specific clinical screening tools, anthropometric and body composition measurements, laboratory tests, a review of clinical data, and examination of an individual’s diet history. Each of these methods has benefits and drawbacks; thus, a combination of assessments may be necessary to provide a more accurate picture of a patient’s nutritional status, especially if nutritional risk or undernourishment are identified. In such cases, a comprehensive nutritional assessment should be undertaken, which includes an evaluation of anthropometric and body composition measurements, laboratory test results, and other clinical data in addition to dietary intake information.
Clinical Screening Tools
A number of screening tools have been developed to identify older adults at risk of malnutrition and undernutrition, including the Mini Nutritional Assessment (MNA),20 Nutrition Risk Screening 2002 (NRS),21 Malnutrition Universal Screening Tool (MUST),22 Short Nutritional Assessment Questionnaire (SNAQ),23 and Subjective Global Assessment (SGA).24 Of these tools, the MNA, which was developed by Vellas and Guigoz in 1989 and published in 1996 in Nutrition Reviews,20 is the most frequently used tool in medical practice and clinical research to determine the nutritional status of individuals 65 years and older, as it has been widely validated to be reliable in this population.
The original MNA is an 18-item questionnaire that takes 10 to 15 minutes to administer. It assesses risk by considering factors such as an individual’s body composition, mobility, lifestyle, arm and calf circumference, weight loss history, eating and drinking habits, medication use, and health and nutritional status perception. Scores between 17 and 23 indicate increased nutritional risk, and scores below 17 reflect malnutrition. The MNA score has been shown to be predictive of mortality, with lower scores correlating with increased mortality risk.25 A major advantage of using the MNA is that it requires no laboratory testing. In addition, a shortened form of the MNA (MNA-SF) is available, which has also been validated and can be completed in <5 minutes, streamlining the screening process.26 The MNA-SF includes six questions that strongly correlate with the original MNA and are predictive of clinical status. These questions examine food intake, weight loss, mobility, psychological stress/acute disease incidence, neuropsychological problems, and BMI or calf circumference. Calf circumference was provided as an option in lieu of BMI, as BMI can be difficult to assess in elders and may inaccurately reflect their nutritional status.27 On the MNA-SF, a score between 8 and 11 points indicates an increased risk of malnutrition, and a score <7 reflects a malnourished state.
Regardless of which nutritional screening tool is used, the frequency with which it is performed needs to be adapted to the setting. In high-risk populations, such as hospitalized elders, nutritional assessments should be conducted more frequently, whereas they can be made less frequently in populations with a decreased risk of malnutrition, such as community-dwelling elders with good functional status. It is recommended that nutritional assessments be performed annually in individuals older than 65 years who reside in the community, and at baseline and every 3 months thereafter for those receiving home care or residing in nursing homes.20,21 Once a patient is identified to be at nutritional risk or already malnourished, a comprehensive assessment to identify reversible causes of malnutrition should be undertaken, such as poor dentition or oral health, gastrointestinal disease, infection, chronic alcohol abuse, depression, use of medications that suppress appetite, social isolation, and limited access to food.
Anthropometric and Body Composition Measures
Anthropometric measures are easy to take and can be an important indicator of an elder’s nutritional status. Obtaining serial body weight measurements can be a useful way to identify a change in overall nutrition status; however, they can be an unreliable indicator in the setting of congestive heart failure, hepatic disorders, or renal disease, as patients with these disorders may have fluid imbalances. When not attributed to rapid fluid shifts, weight loss can be predictive of mortality and is considered clinically significant when there is a >2% decrease in baseline body weight in 1 month, a >5% weight loss in 3 months, or a >10% weight loss in 6 months.13,27 In addition to total body weight, an assessment of fat mass versus lean mass and function should be made. Maintaining lean mass is important to preserve balance and strength and reduce fragility. In clinical practice, BMI is frequently used to determine body fat levels, with a BMI <18.5 kg/m2 indicating underweight and an increased risk of mortality, a BMI of 18.5 to 24.9 kg/m2 indicating normal weight, a BMI of 25 to 29.9 kg/m2 indicating overweight, and a BMI ≥30 kg/m2 indicating obesity. In elders, use of BMI as a nutritional assessment tool may be problematic, as an inaccurate height may be obtained and BMI does not accurately predict body composition. In this population, BMI may underestimate body fat, particularly among those who have reduced muscle mass. Inaccurate heights may be obtained due to vertebral collapse, change in posture, and loss of muscle tone. In these cases, height estimates should be obtained using other methods, such as by measuring knee height or arm span.28
When weights and weight history are difficult to obtain, taking skinfold measures and assessing the circumferences of the extremities are alternative approaches to determining body composition and dimensions. The triceps skinfold (TSF) is reflective of fat stores. To perform this measure, patients bend their elbow at a 90-degree angle while the clinician marks mid-point between the acromion process and the olecranon process. Patients then relax their arm loosely at their side. Using a skinfold caliper, the clinician then palpates the site to distinguish fat from muscle, grasps a fold of skin approximately 1 cm above the mark, and performs three readings (recorded in millimeters); the average of these readings is used. Nutritional depletion (less than the tenth percentile) is defined as a skinfold measure of <11.3 mm in women and <4.3 mm in men.29 A drawback of the TSF is its lack of sensitivity in detecting malnutrition, as some normal adults have a body fat percentage <5%.30
Mid-upper arm circumference (MUAC) is a helpful indicator of malnutrition in ill patients (normal is >21.8 cm in men and >22.3 cm in women29) and has been shown to be an independent predictor of mortality in elders living in long-term care institutions.31 To determine MUAC, the mid-point of the upper arm should be marked as described for the TSF test, and the measuring tape should be snug against the skin but not pinch it. Once TSF and MUAC are taken, a mid-arm muscle circumference (MAMC), an indicator of lean mass, can be calculated using the following equation: MAMC=AC-(TSF x 0.314).29 Individuals are considered nutritionally depleted when their measure falls below the tenth percentile, which is <17.2 cm in women and <19.6 cm in men.
In addition to MAMC, another sensitive indicator of lean body mass is calf circumference. When taking this measurement, the patient should be in the supine position, raise his or her left knee to a right angle between the thigh and calf, and the measuring tape placed around the calf at the greatest circumference without compressing the subcutaneous fat.32 A calf circumference of <31 cm has been shown to correlate with muscle-related disability and self-reported physical function in elderly men and women.33 Another simple tool to measure muscle function is handgrip strength, which strongly correlates with lower extremity muscle power, knee extension torque, and calf cross-sectional muscle area. Decreased handgrip strength is a clinical marker of poor mobility and is a better predictor of muscle function compared with measures of muscle mass.6 Performing serial measurements of BMI in combination with at least one of the indices of lean body mass (MAMC or calf circumference) and muscle strength (eg, handgrip) at baseline and every 3 months in high-risk elders can help identify a change in nutritional status.
Another inexpensive, quick, and noninvasive tool that is widely used in clinical practice to estimate fat mass versus lean mass is bioelectrical impedance analysis (BIA). BIA-enabled devices send a low current through the body to determine the electrical impedance of body tissues, which provides an estimate of total body water (TBW). TBW measures are then plugged into an equation that can determine body composition. This method is appropriate for patients who are ambulatory or bedridden and correlates well with body composition estimates obtained with magnetic resonance imaging.6 Among elders, 27.6% to 34.4% body fat for women and 20.3% to 26.7% for men is considered ideal. BIA does have numerous limitations, however: (1) this method is not useful in patients who have major disturbances of water distribution, such as obese individuals; (2) many factors can affect BIA values, including body position, hydration status, recent consumption of food and beverages, ambient air and skin temperature, recent physical activity, and conductance of the examining table; (3) BIA values depend on proper use and accuracy of the BIA device; and (4) formulas used to estimate fat mass from BIA measures often require height and weight measurements, which can be difficult to obtain in elders, as previously noted in the discussion on BMI.34
Laboratory Assessments
Laboratory assessments are another component of a comprehensive nutritional assessment. Although serum proteins, such as albumin, transthyretin (also called prealbumin), and transferrin, are widely used to assess nutritional status, their levels are impacted by non-nutritional factors. Proteins are significantly influenced by cellular processes, including inflammation, and by hepatic and renal disease; therefore, their use in definitively establishing nutritional status in elderly persons is limited.13 Total lymphocyte count (TLC) has also been used as a marker of nutritional status, but there is little evidence that low TLC levels reflect malnutrition in elders.35 In cross-sectional studies, low total cholesterol (<150 mg/dL) is often observed among individuals with poor nutrition. A total cholesterol level <150 mg/dL in a setting where cholesterol-lowering medications are not being used should alert the physician to perform a more detailed nutritional assessment. Given the limitations of currently used laboratory parameters, there is a need for more sensitive and specific predictors of nutritional status among elders. Two studies evaluated the use of leptin as a clinical predictor of nutritional status in elderly patients.36,37 Leptin levels decrease as malnutrition becomes more pronounced in elderly persons. In both studies, leptin levels were found to have significant positive correlations with weight and all anthropometric measures, but not to the inflammatory marker C-reactive protein. It is postulated that decreased insulin levels may result in reduced leptin production by adipocytes during starvation.36 Another proposed mechanism is that leptin concentration reflects the metabolic reserve constituted by fat, so that leptin levels decrease as the percentage of body fat decreases.37 Bouillanne and colleagues37 suggests an optimal leptin cutoff of 4 µg/L in men (sensitivity, 0.89; specificity, 0.82) and 6.48 µg/L in women (sensitivity, 0.90; specificity, 0.83). Although currently used primarily in research, once additional studies confirm its utility, clinicians may begin to use leptin as a biochemical marker of nutritional status.
Clinical Data Review
Clinical data specific to the nutritional assessment in elders include a review of current medications and their potential impact on appetite, nausea, and mouth dryness; evaluation of oral and swallowing problems; review of gastrointestinal symptoms, such as nausea, vomiting, abdominal pain, diarrhea, and constipation; and review of psychiatric and neurologic disorders.19 The medication review is especially important, as 30% of prescriptions and 40% of over-the-counter medications in the United States are sold to elders.38 Commonly prescribed medications can contribute to malnutrition through multiple mechanisms, including suppressing appetite, altering taste and smell, or by causing malabsorption of nutrients. Medications that can alter taste and smell include lipid-lowering agents, antimicrobials, anti-inflammatory drugs, bronchodilators and other asthma medications, antihypertensives, muscle relaxants, antidepressants, vasodilators, and antihistamines. Medications that cause nutritional deficiencies include lipid-lowering agents, cardiac glycosides, diuretics, antacids, anti-inflammatory agents, and laxatives.39
Diet History Review
The final component of the nutritional assessment is an evaluation of the patient’s diet history. Numerous strategies can be used to gauge a patient’s diet history, including having him or her keep a food diary, conducting a 24-hour recall, and use of various questionnaires to determine the frequency with which foods from various food groups are consumed.40 All of these methods have limitations and drawbacks, but when one of these strategies is used in combination with the aforementioned assessment strategies, useful information on an individual’s diet may be gleaned.
Food Diary. When using a food diary, the patient records everything he or she consumes and the amounts consumed over the course of 1 to 4 days. Having patients log their food and beverage intake for more than 4 days is not recommended, as this task becomes increasingly burdensome over time, leading to a higher rate of inaccuracy.40 Although numerous tools are available to facilitate food logging, including various software and recording instruments, elders are unlikely to have the ability or resources to benefit from such technologies. Another drawback to this method is that it requires the patient to have the ability to adequately describe the items and amounts consumed, as well as their preparation method, which may require a bit of training.
24-hour Recall. The 24-hour recall requires the patient to recount everything he or she consumed over the past 24 hours. Generally, this method requires the interviewer to know how to probe the patient for information, as most information is obtained this way,40 and the patient must have the cognitive ability to recall the items he or she consumed. One of the most widely used 24-hour dietary recall instruments is the US Department of Agriculture’s (USDA) Automated Multiple Pass Method (AMPM), a “research-based, multiple-pass approach employing five steps designed to enhance complete and accurate food recall and reduce respondent burden.”41 Information collected by this tool includes a complete description of the items consumed, including any additions to or combinations of food (eg, milk on cereal), amount consumed, when eaten, name of eating occasion (eg, breakfast, snack), and where obtained and eaten; amount of water consumed; use of salt; whether the amount eaten was a typical or atypical representation of the respondent’s normal diet; and whether the respondent is on any special diets to achieve a health objective.
Food Frequency. The food frequency approach assesses the frequency with which respondents consume foods from a list of foods over a specific period of time. Although portion size may be considered in the assessment, generally few other details of the respondents’ food consumption are collected, making it a much less comprehensive evaluation than the 24-hour dietary recall,40 especially when the latter approach applies the USDA’s AMPM. Some of the food frequency questionnaires commonly used in the United States include the Health Habits and History Questionnaire, Fred Hutchinson Cancer Research Center Food Frequency Questionnaire, Block Food Frequency Questionnaire, Harvard University Food Frequency Questionnaire, and the Willett Questionnaire.40 A discussion of these questionnaires is beyond the scope of this article.
Strategies to Improve Nutrition
Strategies to improve nutrition in older adults include educating them on diet and providing various supplements, including vitamins, minerals, and meal replacements, as needed. An illustrative tool that can be used to demonstrate what constitutes healthy eating and portion size is the USDA’s MyPlate method, which divides a plate into quarters, with one-fourth appropriated to grains, one-fourth to protein, and the remaining half to fruits and vegetables, with dairy on the side (Figure). Patients should be advised that their nutrient intake may be inadequate if they are not eating close to this food composition.

Figure. Illustration showing the allocation of food groups and portions using the MyPlate method to ensure healthful eating. Source: US Department of Agriculture.
If a more specific food guideline is requested or required to meet a health objective, the Dietary Approaches to Stop Hypertension diet, also known as the DASH diet, may be a good option. This diet, which was published as part of the USDA’s 2010 Dietary Guidelines for Americans, has been reported to lower blood pressure and cholesterol levels, and is associated with a lowered risk of several types of cancer, heart disease, stroke, kidney stones, and diabetes.42 On this diet, a nutritionally adequate 2000-calorie plan consists of six to eight daily servings of grains; four to five daily servings of vegetables; five to six daily servings of fruit; two to three daily servings of milk or dairy; 6 oz of lean meat, fish, or poultry daily; and four to five servings per week of nuts, beans, or seeds.
Even if an elder consumes a healthy diet, such as by following the MyPlate method or the DASH diet, the efficiency of nutrient absorption reduces with age, and multivitamin supplementation may be necessary to ensure adequate intake of micronutrients. A 2011 study showed that many older adults are deficient in several essential vitamins,43 including individuals who appear to be otherwise healthy. Nutrients that are especially essential to maintain in elders are protein and vitamin D, as deficiencies in these nutrients have been associated with a higher risk of falls in this population.44,45 Zoltick and colleagues44 examined food intake in 807 men and women, aged 67 to 93 years, from the Framingham Original Cohort Study, and found that higher protein intake was associated with decreased odds of falling. In those with a >5% weight loss from baseline, higher protein intake significantly decreased fall incidents. The researchers concluded that protein intake may be a modifiable factor for fall prevention in elders.44
There has been recent debate about the adequacy of the RDA for protein in older adults. The current Institute of Medicine recommendation for all men and women aged 19 years and older is 0.8 g per kilogram of body weight daily, which was based on short-duration nitrogen balance studies in young adults.46 The question is whether this level of protein is adequate to protect elders from muscle loss.47 The Health, Aging, and Body Composition Study48 found that over a 3-year period, community-dwelling elders who had the highest intake of protein (average of 1.2 g/kg) lost about 40% less lean mass than those with the lowest intake. Paddon-Jones and Rasmussen49 recommend 25 to 30 g of protein (3.5-4 oz) at each meal to maximize muscle protein synthesis. This equates to about 75 to 90 g of protein per day for a 154-lb man. Additional research is needed to ascertain the optimal protein recommendation.
A recent systematic review and meta-analysis of older adults (>60 years) investigated the effectiveness of vitamin D in the prevention of falls.45 The authors report that vitamin D therapy (200-1000 IU) results in 14% fewer falls. Although the optimal daily dose of vitamin D continues to be debated, the authors conclude that vitamin D supplementation is an effective strategy for reducing falls in older adults. In “Vitamin D and Calcium: Implications for Healthy Aging,” a previous article in this nutrition series, we reviewed the current data and recommendations regarding calcium and vitamin D in elders; the article is available at www.clinicalgeriatrics.com/node/4494.
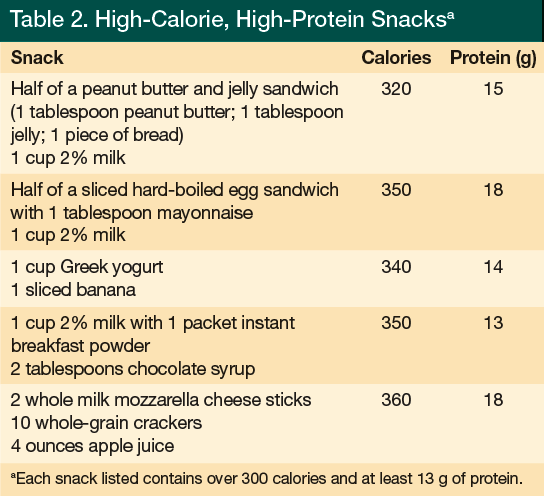
When a nutritional assessment reveals that an elder has an inadequate energy intake, one approach to remedy this is to offer nutritionally complete supplements with a nutrient composition between 1.0 and 1.5 calories per milliliter and a protein content in the range of 15% to 20% of total calories. A 2009 Cochrane Review50 concluded that oral supplementation produces small but consistent weight gain in older adults and that mortality may be reduced among older undernourished persons who regularly consume complete nutritional supplements. However, the review found no evidence of improvement in functional status or reduction in length of hospital stay with oral supplements, and it concluded that additional data from large-scale multicenter trials are still needed. The benefit of offering a commercial supplement is that a uniform composition of macronutrients, vitamins, and minerals can be provided. In addition, oral supplements may be of particular benefit for those individuals living at home who may have difficulty shopping or physically preparing food.5 When offering oral supplements to the elderly population, it may be useful to offer a less sweet product, as sweetness has been identified as a major deterrent to supplement acceptance. A study by Kennedy and colleagues51 found that chocolate was the most liked flavor and was perceived as less sweet. Although commercial supplements may be beneficial, there is nothing magical about their nutrient composition. Calories and protein can be provided by making homemade milkshakes using powdered products, such as instant breakfast, or by adding high-calorie and high-protein foods to the diet (Table 2). Whether oral supplements or additional food is used to increase intake, compliance monitoring is key to ensuring adequacy of the intervention.
In some cases, it may be necessary to refer patients to other healthcare providers after a nutritional assessment is completed. A speech-language pathologist can be invaluable in assessing and treating swallowing problems. A registered dietitian can be of value in the assessment and treatment of reversible nutrition issues, such as by determining the adequacy of intake, developing a custom meal plan, making recommendations for increasing oral intake, or developing a plan to initiate nutrition support. A social worker can assist patients and families to obtain access to home-delivered meals, congregate feeding programs, food stamps, and other assistance programs. A referral to a dentist for oral health issues or to a geriatric psychiatrist for mental health issues may be warranted. An occupational or physical therapist consultation may be of value for patients with physical limitations. Finally, a pharmacist can provide assistance with nutrition-related medication management issues.
Conclusion
Malnutrition in the elderly is a multifaceted and complex issue. No single tool or clinical marker accurately predicts nutritional status. We recommend that the initial assessment be made using the MNA or a similar screening tool in combination with a total cholesterol level assessment. By integrating a validated nutrition screening tool with anthropometric and laboratory data, the geriatric practitioner can get a more accurate picture of a patient’s nutritional status. When reversible causes of malnourishment are identified, appropriate interventions should be undertaken, which may require referrals to other disciplines, including to a registered dietician.
Dr. Steinle receives salary support in part from the Mid-Atlantic Nutrition Obesity Research Center (NORC), University of Maryland School of Medicine - NIH Grant P30 DK072488. The other authors report no relevant financial relationships.
References
1. Kaiser MJ, Bauer JM, Rämsch C, et al; Mini Nutritional Assessment International Group. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738.
2. Reuben DB. Quality indicators for the care of undernutrition in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S438-S442.
3. Mokdad AH, Bowman BA, Ford ED, Vinicor F, Mark JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195-2000.
4. Chapman IM. Obesity in old age. http://bit.ly/GNM5QA. Accessed March 28, 2012.
5. Stratton RJ, Hackston A, Longmore D, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92(5):799-808.
6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia. Age Ageing. 2010;39(4):412-423.
7. Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26(4):389-399.
8. Mosby’s Medical Dictionary. Overnutrition. http://medical-dictionary.thefreedictionary.com/overnutrition. Accessed March 28, 2012.
9. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO; The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82(5):923-934.
10. Wham CA, Teh Ro, Robinson M, Kerse NM. What is associated with nutrition risk in very old age? J Nutr Health Aging. 2011;15(4):247-251.
11. de Castro JM. Age-related changes in the social, psychological, and temporal influences on food intake in free-living, healthy, adult humans. J Gerontol A Biol Sci Med Sci. 2002;57(6):M368-M377.
12. German L, Feldblum I, Bilenko N, Castel H, Harman-Boehm I, Shahar DR. Depressive symptoms and risk for malnutrition among hospitalized elderly people. J Nutr Health Aging. 2008;12(5):313-318.
13. Morley JE. Assessment of malnutrition in older persons: a focus on the Mini Nutritional Assessment. J Nutr Health Aging. 2011;15(2):87-90.
14. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141-147.
15. Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864-3871.
16. Chipperfield JG. Everyday physical activity as a predictor of late-life mortality. Gerontologist. 2008;48(3):349-357.
17. Dorner B, Friedrich EK, Posthauer ME; American Dietetic Association. Position of the American Dietetic Association: Individualized nutrition approaches for older adults in health care communities [published correction appears in J Am Diet Assoc. 2010;110(12):1941]. J Am Diet Assoc. 2010;110(10):1549-1553.
18. Locher JL, Ritchie CS, Roth DL, Sen B, Vickers KS, Vailas LI. Food choice among homebound older adults: motivations and perceived barriers. J Nutr Health Aging. 2009;13(8):659-664.
19. Bauer JM, Kaiser MJ, Sieber CC. Evaluation of nutritional status in older persons: Nutritional screening and assessment. Curr Opin Clin Nutr Metab Care. 2010;13(1):8-13.
20. Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(1 pt 2):S59-S65.
21. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Education and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415-421.
22. Elia M; British Association for Parenteral and Enteral Nutrition. The ‘MUST’ Report. Nutritional Screening of Adults: A Multidisciplinary Responsibility. Development and Use of the ‘Malnutrition Universal Screen Tool (‘MUST’) for Adults. Redditch, England: BAPEN, 2003.
23. Kruizenga HM, Seidell JC, de Vet HCW, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr. 2005;2(1):75-82.
24. Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8-13.
25. Chan M, Lim YP, Ernest A, Tan TL. Nutritional assessment in an Asian nursing home and its association with mortality. J Nutr Health Aging. 2010;14(1):23-28.
26. Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. 2001;56(6):M366-M372.
27. Kaiser MJ, Bauer JM, Ramsch C, et al; MNA-International Group. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788.
28. Hickson M, Frost G. A comparison of three methods for estimating height in the acutely ill elderly population. J Hum Nutr Diet. 2003;16(1):13-20.
29. Burr ML, Phillips KM. Anthropometric norms in the elderly. Br J Nutr. 1984;51(2):165-169.
30. RxKinetics. Nutritional assessment. http://www.rxkinetics.com/tpntutorial/1_3.html. Accessed April 3, 2012.
31. Allard JP, Aghdassi E, McArthur M, et al. Nutrition risk factors for the survival in elderly living in Canadian long-term care facilities. J Am Geriatr Soc. 2004;52(1):59-65.
32. Bonnefoy M, Jauffret M, Kostka T, Jusot JF. Usefulness of calf circumference measurement in assessing the nutritional state of hospitalized elderly patients. Gerontology. 2002;48(3):162-169.
33. Rolland Y, Lauwers-Cances V, Cournot M, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51(8):1120-1124.
34. National Institutes of Health. Bioelectrical Impedance Analysis in Body Composition Measurement. http://consensus.nih.gov/1994/1994BioelectricImpedanceBodyta015html.htm. Accessed April 3, 2012.
35. Kuzuya M, Kanda S, Koike T, Suzuki Y, Iguchi A. Lack of correlation between total lymphocyte count and nutritional status in the elderly. Clin Nutr. 2005;24(3):427-432.
36. Amirkalali B, Sharifi F, Fakhrzadeh H, et al. Low serum leptin serves as a biomarker of malnutrition in elderly patients. Nutr Res. 2010;30(5):314-319.
37. Bouillanne O, Golmard JL, Coussieu C, et al. Leptin a new biological marker for evaluating malnutrition in elderly patients. Eur J Clin Nutr. 2007;61(5):647-654.
38. Conry M. Polypharmacy: pandora’s medicine chest? Geriatric Times. 2005;1(3).
www.cmellc.com/geriatrictimes/g001028.html. Accessed April 6, 2012.
39. Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S54-S63.
40. Thompson FE, Subar AF; National Cancer Institute. Dietary assessment methodology. http://bit.ly/GGUSEJ. Accessed April 3, 2012.
41. United States Department of Agriculture. USDA Automated Multiple-Pass Method. www.ars.usda.gov/services/docs.htm?docid=7710. Accessed April 3, 2012.
42. The DASH diet eating plan. http://dashdiet.org/. Accessed April 3, 2012.
43. Toffanello ED, Inelmen EM, Minicuci N, et al. Ten-year trends in vitamin intake in free-living healthy elderly people: the risk of subclinical malnutrition. J Nutr Health Aging. 2011;15(2):99-103.
44. Zoltick ES, Sahni S, McLean RR, Quach L, Casey VA, Hannan MT. Dietary protein intake and subsequent falls in older men and women: the Framingham study. J Nutr Health Aging. 2011;15(2):147-152.
45. Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: Systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1299-1310.
46. Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77(1):109-127.
47. Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept [published correction appears in JAMA. 2008;300(15):1763]. JAMA. 2008;299(24):2891-2893.
48. Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) study. Am J Clin Nutr. 2008;87(1):150-155.
49. Paddon-Jones D, Rasmussen B. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86-90.
50. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;15(2):CD003288.
51. Kennedy O, Law C, Methven L, Mottram D, Gosney M. Investigating age-related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing. 2010;39(6):733-738.


