Noncancer Pain Management in Seriously Ill Older Adults: Practical Considerations With Opioid Use
Key words: Noncancer pain, pain management, pain control, opioid use in the elderly.
_______________________________________________________________________________________
Noncancer pain is a common experience reported by older adults. The prevalence of pain varies by setting, with 30% to 50% of community-dwelling older adults and up to 80% of nursing home residents reporting moderate to severe pain.1-3 The high frequency of pain experienced by older persons has been attributed to an increasing number of comorbid conditions that occur with aging and are associated with both nociceptive and neuropathic pain.4-6 Osteoarthritis is the most common source of nociceptive pain, affecting 50% of older adults, with almost 50% of these individuals experiencing arthritis-attributable activity limitations.7 Moreover, several sites are often affected, compounding activity limitations and pain-related suffering. Common sources of neuropathic pain in older persons include diabetic neuropathy and postherpetic neuralgia.
In addition to discomfort, noncancer pain is associated with worse outcomes compared with those observed in persons who do not have pain, and may include greater functional impairment, depression, decreased appetite, and impaired sleep.8-10 As a result, it is not surprising that older adults with persistent pain report a poorer self-rated health status than those without pain. Recent research suggests that persistent pain may precipitate or accelerate the development of frailty because the multidimensional impact of pain may leave older adults more vulnerable to and less able to effectively accommodate physiologic stressors.11
Although pain can be controlled in most patients, it remains undertreated.12,13 Myths frequently held by older adults may contribute to this undertreatment, including the expectation that pain is a normal part of aging, a reluctance to report pain based on the previous dismissal of symptoms by healthcare providers, or the fear of having an opioid prescribed.8 Older adults at the highest risk for inadequate analgesia include the oldest old (persons ≥85 years), ethnic minorities (especially African-Americans), and those with cognitive impairment.14
The goals of pain management should be discussed at the initiation of therapy. Clinicians must communicate to the patient that substantial and sustainable improvements in pain are attainable, but that the complete elimination of pain is typically not achievable. In this review, we discuss considerations for the safe introduction and monitoring of opioid therapy in older adults with pain. We highlight the unique characteristics of commonly used opioids, provide recommendations for starting doses in opioid-naïve older adults, consider the impact of age and illness on opioid pharmacokinetics and pharmacodynamics, and review opioid-related side effects and approaches for managing them effectively.
General Guidelines for Assessing and Managing Pain in Elders
The first critical step in pain management is adequate assessment.15 Patient self-report remains the gold standard, and clinicians must complete a comprehensive assessment that includes the quantification of pain intensity along with a description of its location, quality, and aggravating and alleviating factors. Before initiating a pain assessment, providers should determine a patient’s preferred terminology, because older adults may deny pain and use terms such as achiness, discomfort, or soreness instead. A hierarchical approach to pain assessment is strongly encouraged, which includes patient self-report using a validated scale, such as the Iowa Pain Thermometer16 or the Faces Pain Scale–Revised.17 In addition to assessing pain intensity, clinicians should evaluate the impact of pain on function and quality of life. When self-report is not possible (eg, patient has advanced dementia or is nonverbal) or its accuracy is questioned, the presence of painful conditions (eg, gout, osteoarthritis, fracture) should be evaluated for. At the same time, pain behaviors (eg, facial expressions, vocalizations, body movements, changes in interpersonal interactions or activity, mental status changes) should be assessed for. More formalized pain assessment tools for nonverbal patients may be helpful, with experts18 generally recommending the Pain Assessment in Advanced Dementia Scale19 or the Pain Assessment Checklist for Senior with Limited Ability to Communicate.20 A proxy report of patient pain from a professional or family member can also provide valuable information about a patient’s pain experience. Finally, an empiric analgesic trial can be considered to evaluate whether or not pain or the pain behavior responds to treatment.
Pain management is most effective when the underlying cause is identified and appropriately treated; however, in older adults, several concomitant etiologies may contribute to a painful condition. For example, an older adult may present with an acute worsening of knee pain secondary to a pseudogout flare with underlying knee osteoarthritis from a leg-length discrepancy that has not been adequately managed, along with quadriceps muscle weakness secondary to disuse. Therefore, a complete medical history can help uncover potential contributing factors. The impact of pain on patients’ function, mood, and relationships with family and friends should be explored, as well as previously used effective coping strategies, social support systems, and general beliefs about pain and its treatment. Finally, a thorough review of previously attempted management strategies should be ascertained and documented, including all prescription and over-the-counter medications used, dosages, and levels of effectiveness.
Guidelines for Treating Pain
General principles of noncancer pain management are well-established and supported by several consensus guidelines, including those from the American Pain Society and the American Geriatrics Society.8,15 As part of a comprehensive treatment plan, analgesics are often considered to decrease pain intensity and to help improve a patient’s well being.21 The World Health Organization’s three-step pain ladder, initially developed as an approach for managing cancer-related pain, has been widely accepted and adopted as a guide for selecting analgesics for noncancer pain.8 The underlying premise of the ladder is that pain intensity guides analgesic selection. Step 1 corresponds to mild-intensity pain and includes the use of acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), or both. Step 2 denotes moderate pain and promotes the use of “mild opioids,” generally considered to be combination products, such as acetaminophen or an NSAID added to an opioid or tramadol. Step 3 represents severe pain and suggests the use of “strong opioids,” such as morphine, oxycodone, and hydromorphone. Step-1 therapies should continue to be used in conjunction with step-2 and step-3 analgesics. Coanalgesics should be considered with each step, with analgesic selection based on the underlying etiology of the pain. For instance, an older adult with moderate to severe pain secondary to postherpetic neuralgia may be treated first with gabapentin, with the subsequent addition of a combination opioid, such as oxycodone plus acetaminophen.22
The adoption of guidelines including opioids for the management of moderate to severe noncancer pain in older adults has lead to a dramatic increase in their use over the past decade.23 This approach is supported by research that opioid use in older adults is associated with decreased pain intensity and improved function.21 These benefits must be tempered with safety concerns, which have been recently highlighted.24,25 The physiologic changes that accompany normal aging, coupled with the complications of serious illness, add a level of complexity to opioid treatment that requires individualized approaches and consideration of drug-drug and drug-disease interactions. Despite these challenges, opioids serve an important therapeutic role in managing noncancer pain in older adults with serious illnesses.
Characteristics of Commonly Used Opioids
Tremendous variability exists between individuals in response to a particular opioid, including in its analgesic effects and side effects.26 Despite this, randomized controlled trials do not demonstrate significant short-term differences in efficacy or adverse effects among different opioids.27 A better understanding of the unique characteristics of commonly used opioids can help clinicians select the most appropriate agent for each older patient. One important difference between opioids is expense, with newer high-cost agents placing an undue financial burden on patients and families, without providing any additional therapeutic benefits. What follows is a review of some of the unique characteristics of commonly prescribed opioids in older persons, which are also outlined in Table 1. An additional discussion about opioid metabolites appears in the section on opioid pharmacokinetics and pharmacodynamics.
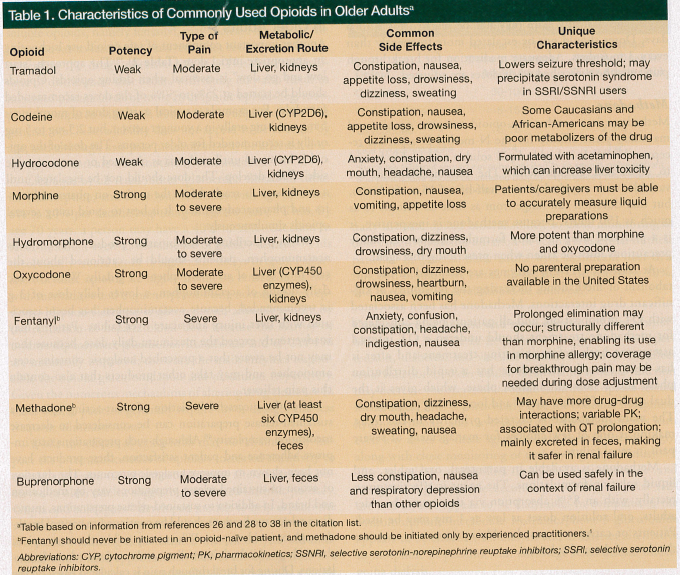
Tramadol
Tramadol is a weak opioid agonist and a mild inhibitor of serotonin and norepinephrine reuptake. It is 10% as potent as morphine.26,28,29 Tramadol is metabolized in the liver to one active metabolite, O-desmethyltramadol, which provides most of the µ-opioid activity, and 90% of the drug is excreted renally.28 The side-effect profile is similar to that of other opioid analgesics (attributable to its mu activity), but this agent has additional concerns for older populations. One concern is that it lowers the seizure threshold, especially when given at higher doses.26 Therefore, particular caution should be exercised in older adults at higher risk of seizures, such as those with dementia or underlying cerebrovascular disease, or in older adults taking other medications known to lower the seizure threshold (eg, antipsychotics). Tramadol should be avoided in persons taking antidepressant agents that are selective serontonin reuptake inhibitors (SSRIs) or selective serotonin-norepinephrine reuptake inhibitors (SSNRIs) because of the potential for precipitating serotonin syndrome. The maximum daily dose of tramadol is 400 mg daily.26
Codeine
Codeine is a weak opioid agonist indicated for moderate pain.28,29 It is available in parenteral and oral formulations and is most commonly prescribed as a combination product with acetaminophen. Codeine is a prodrug and must be metabolized to its active metabolites to be effective. The analgesic effect of codeine is primarily through conversion to morphine-3-glucuronide. Approximately 5% is converted to morphine via oxidation in the liver. Genetic variations in the cytochrome pigment (CYP) 2D6 enzyme can lead to either reduced efficacy in poor metabolizers (predominately those of Caucasian and African-American descent, comprising 7%-10% of the population) or overmedication in rapid metabolizers. Several medications commonly used in geriatrics reduce or block the CYP2D6 conversion of codeine; these include the SSRIs paroxetine and fluoxetine as well as the antihistamine diphenhydramine.30 Patients taking these drugs should not be prescribed codeine-containing products.
Hydrocodone
Hydrocodone is a semisynthetic opioid agonist indicated for moderate pain.28,29 It is available only in combination with other ingredients, such as acetaminophen and ibuprofen, and comes in tablet, capsule, or liquid formulations. Dosing is limited by the amount of the nonopioid medication the compound contains (eg, acetaminophen). Hydrocodone itself is a weak opioid, but it is metabolized by CYP2D6 to the more potent hydromorphone. One common pitfall in opioid prescribing is the underestimation of opioid consumption. For example, when the 10 mg hydrocodone/325 mg acetaminophen combination product is prescribed, patients may take up to 12 tablets daily before concerns of acetaminophen toxicity arise, but that represents 120 mg of hydrocodone or approximately 120 mg of morphine equivalence. Patients taking this much hydrocodone are at risk of developing withdrawal symptoms if the medication is discontinued abruptly, especially when a patient transitions from one care setting to another (eg, home to hospital).
Morphine
Morphine is the prototypical opioid.28,29,31 It is available in many formulations, including as oral immediate-release and extended-release tablets, a liquid in several concentrations, and parenteral preparations. The lowest dose of the immediate-release tablet is 15 mg. When a lower starting dose is needed, a liquid preparation must be used and dosed with a dropper. The patient or caregiver must be able to properly measure the medication based on the concentration of the liquid.
Hydromorphone
Hydromorphone is a semisynthetic opioid, which is a derivative of morphine that is five to ten times more potent than morphine.28,29,31,32 Hydromorphone is metabolized to hydromorphone-3-glucuronide, which is a neuroexcitant 2.5 times more potent than morphine-3-glucuronide and can also accumulate in the presence of renal insufficiency. Hydromorphone is available in parenteral and immediate-release oral preparations. Although an extended-release preparation was available in the United States, it has been withdrawn from the market. The extended-release form remains available in Europe.
Oxycodone
Oxycodone is a semisynthetic opioid with high oral bioavailability.28,29,31,33 It is available in oral immediate-release and controlled-release tablets and in a concentrated solution. It is also available in combination with acetaminophen and aspirin. No parenteral formulations are available in the United States.
Fentanyl
Fentanyl is a synthetic opioid that is 80 to 100 times more potent than morphine. As a piperidine, it is completely different structurally from morphine; thus, it can be used in the case of a true morphine allergy.28,29,31,34 Fentanyl is available in parenteral, transdermal, and immediate-release formulations, including a lozenge on a stick, buccal dissolvable tablets, and as a nasal spray. Fentanyl is metabolized by the liver, primarily to norfentanyl (>99%) and other inactive metabolites. Because fentanyl is highly lipophilic and highly protein bound, prolonged elimination can occur in older adults. Transdermal fentanyl should never be started in an opioid-naïve individual. When initiating transdermal fentanyl, it can take 18 to 24 hours to reach peak plasma levels, and a steady state may not be reached for 3 to 6 days. Doses should not be escalated more frequently than every 3 to 6 days. Adequate medication for breakthrough pain must be prescribed while doses are adjusted.
Methadone
Methadone is a synthetic µ-opioid receptor agonist and maintains some affinity for the N-methyl-D-aspartate receptor.28,29,31,35 It is highly lipid soluble and highly bound to alpha-acid glycoproteins. The pharmacokinetics are variable, with a typical plasma half-life of 15 to 60 hours, but that duration can range from as little as 4 hours to as much as 190 hours. Because methadone is inexpensive, it is a preferred drug in many formularies; however, many precautions must be taken when prescribing this agent.
At least six CYP450 enzymes are involved in the metabolism of methadone, increasing the possibility of significant drug interactions. Methadone has been associated with QT prolongation, and all patients being considered for methadone treatment should undergo a QT interval assessment both before initiating treatment and after it has been started. Methadone has a rapid distribution phase and a slow elimination phase, which gives it the dual quality of both a short- and long-acting preparation. The main metabolite is excreted primarily in the feces, making it one of few choices for management of severe pain in renal failure.
Methadone is available in parenteral, oral tablet, and liquid solution formulations. The agent is effective given rectally, with an 85% absorption via that route. In older adults, oral solution doses as low as 1 mg may be used. Patients or caregivers must be able to accurately measure the dose of the oral solution.
Buprenorphine
Buprenorphine, a semisynthetic opioid with partial agonist and antagonist actions, is now available in parenteral, sublingual, and transdermal formulations.31,36 Although it was previously thought to have an analgesic ceiling effect (ie, increased dose above a certain amount does not result in additional analgesia), more recent evidence calls this idea into question. It may have no ceiling effect for analgesia, but it does have a ceiling effect for respiratory depression.37 It also appears to produce less constipation and nausea compared with other opioids. Although there are no studies specifically designed to evaluate its use in older adults, safety and efficacy was shown in a study of 3690 patients with cancer, 44% of who were older than 70 years.38 Buprenorphine can be used safely in renal failure because it is mainly excreted by the liver.38 In the United States, prescription of this medication requires a Risk Evaluation and Mitigation Strategy.
Starting Doses for Commonly Used Opioids in Older Adults
 Another important component of safe opioid use is choosing an appropriate initial dose (Table 2).39 The approach “start low and go slow” is essential when dosing opioids. Opioids should be started at 25% to 50% of the doses recommended for adults. For example, a typical starting dose of morphine is 5 mg to 10 mg orally in a younger person, but 2.5 mg to 5 mg orally is recommended for older persons. The dose of the opioid is increased until analgesia is obtained or unmanageable side effects develop. The dose should not be escalated until a steady state is reached (see the section on pharmacokinetics and pharmacodynamics). It is best to avoid using several opioids simultaneously.
Another important component of safe opioid use is choosing an appropriate initial dose (Table 2).39 The approach “start low and go slow” is essential when dosing opioids. Opioids should be started at 25% to 50% of the doses recommended for adults. For example, a typical starting dose of morphine is 5 mg to 10 mg orally in a younger person, but 2.5 mg to 5 mg orally is recommended for older persons. The dose of the opioid is increased until analgesia is obtained or unmanageable side effects develop. The dose should not be escalated until a steady state is reached (see the section on pharmacokinetics and pharmacodynamics). It is best to avoid using several opioids simultaneously.
When prescribing a combination product that contains acetaminophen, patients should be cautioned about the safe total dose of acetaminophen—4 g daily. With chronic daily dosing of acetaminophen, a lower daily dose of 3 g is recommended, because acetaminophen has been associated with liver injury and acute liver failure. Patients may inadvertently exceed the maximum daily dose, because they may not be aware that a prescribed analgesic contains acetaminophen and may take other products that also contain this pain reliever.
After the successful initiation of short-acting opioids, a sustained-release preparation can be considered to decrease medication complexity.40 Although such preparations may improve adherence and patient satisfaction, these products have not been shown to improve analgesic outcomes. The duration of action of sustained-release preparations vary by medication and brand. In addition to sustained-release preparations, immediate-release medications should be continued to control breakthrough or incident pain. Whenever possible, the same opioid should be used in both immediate- and sustained-release formulations. Dosing for breakthrough pain is calculated based on the total 24-hour sustained-release dosage and is typically 10% of that dosage, given as often as every 4 hours.
Opioid Pharmacokinetics and Pharmacodynamics
Numerous factors can affect the pharmacokinetics and pharmacodynamics of opioids, including factors associated with normal aging, such as a natural decline in organ function, and comorbidities, which are more common in elderly persons. A review of these issues follows.
Effects of Normal Aging
Numerous well-documented pharmacokinetic alterations have been described as a result of the natural decline in the functioning of all organs caused by the normal aging process, which begins in the sixth decade of life.41 Reduced intravascular volume, organ volume, and muscle mass may alter drug distribution, resulting in increased plasma levels relative to that of a younger person. The volume of distribution of fat-soluble opioids, namely fentanyl, may increase because of the increased fat-to-lean body mass ratio that accompanies aging, increasing the drug’s effective half-life. The decreased volume of distribution that occurs with aging may also result in increased plasma levels of more hydrophilic opioids (eg, morphine) compared with levels observed in younger persons.42 In general, oral bioavailability does not appear to be affected by age, and although first-pass metabolism may be affected, dosage adjustments are note routinely recommended.43
Clearance by all renal routes (glomerular filtration, tubular reabsorption, and secretion) decreases with age by about 6% to 10% per decade beginning at age 40 years.44 Thus, by age 70 years, a person may have a 40% to 50% reduction in renal function, even in the absence of kidney disease.43 This theoretically would result in significant delays in opioid clearance, because opioids are highly reliant on renal elimination, with methadone and buprenorphine being noteworthy exceptions. Hepatic clearance is also reduced, affected mainly by the reduction of hepatic blood flow, because the activity of uridine diphosphate glucuronosyltransferase and of CYP450 are minimally impacted; these are the main enzymes responsible for opioid glucuronidation and oxidation, respectively. Given the numerous mechanisms of altering drug clearance in older adults, patients generally require lower dosages and less frequent administration.
While the effects of aging itself may not dramatically impact the pharmacokinetics of opioids, increased sensitivity to opioid analgesics (or pharmacodynamics alterations) are observed in older adults, suggesting an altered intrinsic potency. Gupta and colleagues39 developed models to help explain the relationship between age and the effect of opioids on mu receptors. Modeling demonstrated that an 80-year-old individual would need approximately half of the opioid dose as a 40-year-old to achieve the same effect.39 For example, a single 10-mg dose of morphine in an immediate-release preparation administered to an 80-year-old will provide an approximate 50% increase in peak analgesic effect, maximum respiratory depression, and duration of clinical effect; however, if this dose were reduced by 50%, to 5 mg, it will produce a clinical effect similar to that of a 10-mg dose administered to a 40-year-old. Therefore, given the observed increased sensitivity of older adults to opioid analgesics, a 25% to 50% dose reduction should be considered when prescribing opioids to patients older than 60 years.
Effects of Comorbidities
In addition to the altered intrinsic potency of opioids that are dependent on age, underlying comorbidities must also be considered before initiation of these agents.
Liver dysfunction. Hepatitis, cirrhosis, or hepatic malignancy that significantly affect hepatic function can substantially increase opioid bioavailability, so close monitoring of dose effectiveness and duration of action is essential in these patients. In general, older adults with significant liver dysfunction should have initial opioid doses decreased by 50%, and the dosing interval should be doubled.45
Cardiovascular disease and renal function. Many comorbidities that increase in frequency with age, such as hypertension, diabetes, and vascular disease, can adversely affect renal function in older adults. Decrements in renal function may decrease the excretion of some neurotoxic opioid-related metabolites. This is particularly true for codeine, morphine, hydromorphone, and oxycodone, and dose adjustments with even low doses of these agents should be made accordingly, along with close monitoring of toxicity (eg, myoclonus).46,47 The morphine metabolites morphine-3-glucuronide and morphine-6-glucuronide accumulate rapidly in renal failure and do not appear to be dialyzable. In comparison, the hydromorphone metabolite hydromorphone-3-glucuronide appears to be cleared with dialysis and is a better alternative to morphine in patients with end-stage renal disease undergoing dialysis.
Codeine should be not be used in patients with a creatinine clearance <30 mL/min/1.73m2 because there have been reports of substantial toxicity even with relatively low doses.28 Oxycodone has several active metabolites that may accumulate in renal dysfunction, but is considered to be safer than morphine. Limited case reports and pharmacokinetic data suggest that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in patients undergoing dialysis if the drug’s use is accompanied by proper monitoring of respiratory and cardiovascular status, blood pressure, and heart rate.
Methadone is primarily excreted in feces; thus, it is considered safe for use in persons with renal insufficiency. However, equianalgesic ratios between morphine and methadone are dose-dependent and should be used only by experienced practitioners.35 Buprenorphine is also excreted primarily via feces and is considered safe in persons with renal impairment.36
The starting dose of opioids in older adults is 25% to 50% lower than in younger persons, without a loss of analgesic effect.39 The time of the maximal effect of opioids does not change with aging, so the onset of action of oral preparations is expected to occur in approximately 30 minutes (6-10 minutes intravenously and 30 minutes subcutaneously), reaching peak plasma levels (peak effect) in approximately 1 hour and lasting approximately 3 to 4 hours. However, many experts in geriatric pain management recommend a longer time interval (usually 6 hours) between doses of short-acting preparations at the initiation of opioid therapy, given the heterogeneity in response found in older persons. A steady state is generally reached at around 4 to 5 half-lives of the drug.
Opioid-Related Side Effects and Management Strategies
One common reason cited for noncompliance with opioid therapy is the fear of side effects. The side effects of most concern include constipation, nausea and vomiting, sedation, confusion, and respiratory depression. Tolerance develops to most of these side effects, with constipation being a notable exception. Reassurance to the patient coupled with the effective management of common side effects are key strategies for decreasing complications and increasing adherence to opioid therapy.
Patients’ prescribed opioid analgesics need to be monitored closely with a reevaluation for efficacy and side effects after initiation or titration. Evidence does not exist to guide practitioners on an optimal approach for monitoring patients on opioids. We recommend that providers tailor follow-up to their patients’ needs. For example, an otherwise healthy older adult may only be asked to call the office if an opioid-related side effect occurs or the therapy is not effective; a patient with several comorbidities and an unstable gait may require follow-up in a week in addition to calling the clinician’s office in a couple of days so that efficacy and side effects can be better gauged; and a frail older adult or someone with underlying cognitive impairment may benefit from home nursing services to monitor opioid therapy while also having regular physician follow-up visits to optimize treatment.
When opioid-related side effects emerge, practitioners can employ several strategies, including treatment of the side effect, opioid dose reduction, change in the route of opioid administration, and opioid rotation.48 Treating through the side effect and opioid rotation have the strongest evidence base; thus, these two strategies are focused on here. Both approaches require special consideration in older adults.
Treating Through the Side Effects
Nausea develops in about one-third of patients initiated on opioids and occurs because these agents slow down the motility of the gastrointestinal (GI) tract, stimulate the chemoreceptor trigger zone, and sensitize the vestibular apparatus. This generally resolves within 1 week. Several medication classes are available to counteract these effects, including neuroleptics, selective 5-hydroxytryptamine-3-receptor antagonists, and anticholinergics. Each of these treatments predispose to additional adverse effects, which may be particularly problematic in older adults.
Constipation is common in persons with serious illnesses and nearly universal in patients taking opioids, particularly among older adults who have existing difficulty with elimination. This malady is a common fear cited by older adults and a frequent reason for refusing to start an opioid. Clinicians need to educate and reassure patients about opioid-related constipation and promote prevention. Constipation results from µ-receptor binding in the GI tract, resulting in slower transit time and increased water reabsorption. This side effect should be anticipated and managed preemptively with a combination of a bowel stimulant and a stool softener.
One common pitfall in constipation management is the preferential use of osmotic agents, such as polyethylene glycol or milk of magnesia, which are generally insufficient to counteract the decrease in gut transit time caused by µ-receptor binding. These agents should be used in addition to stimulant laxatives and stool softeners when needed. Bulk-forming agents, such as psyllium, are ineffective and can worsen symptoms if patients do not ingest an adequate amount of fluid.
Opioid Rotation
While opioid rotation avoids polypharmacy, it does expose older adults to another opioid that could have different or more severe side effects. A practical approach is to determine if an older adult has had any previous exposure to opioids, possibly after an acute injury or surgery. The clinician can then determine whether the agent was tolerated and effective and choose an analgesic with the most benefit and fewest side effects.
Excessive sedation may occur with the initiation and escalation of opioid therapy. Tolerance to sedation usually occurs within a few days to a week, but it can persist and necessitate a consideration of opioid rotation in some patients. In patients with cancer, a trial of a stimulant, such as methylphenidate, has been studied and found to be effective. However, stimulants need to be used judiciously in older populations because of concerns about triggering or worsening an atrial tachycardia or psychosis.
Other Considerations
Respiratory depression is rare in opioid-naïve patients whose treatment is initiated at low doses. Risk has been shown to increase with age and with underlying pulmonary conditions, such as chronic obstructive pulmonary disease and sleep apnea. Opioids should be initiated and titrated extremely cautiously in older adults in the acute care setting when an acute pulmonary condition, such as pneumonia or an asthma exacerbation, is present. Finally, the concomitant administration of opioids with other central nervous system depressants (eg, benzodiazepines, alcohol, barbiturates) can increase the risk of respiratory depression.
Conclusion
Managing moderate to severe pain with opioids in seriously ill older adults presents challenges that can be addressed best via a thorough assessment, awareness of the individual variability in opioid response, knowledge of the differences between available opioids, consideration of the pharmacokinetic and pharmacodynamic changes that occur with aging and comorbidities, and an awareness of opioid-related side effects and how to optimally manage them. The narrow therapeutic index of opioids for older persons can be proactively addressed so that patients can benefit from the superior analgesic effect of opioids while minimizing and mitigating adverse effects and risks.
References
1. Maxwell CJ, Dalby DM, Slater M, et al. The prevalence and management of current daily pain among older home care clients. Pain. 2008;138(1):208-216.
2. Jakobsson U, Klevsgard R, Westergren A, Hallberg IR. Old people in pain: a comparative study. J Pain Symptom Manage. 2003;26(1):625-636.
3. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153(9):563-569.
4. Laiho K, Tuohimehto J, Tilvis R. Prevalence of rheumatoid arthritis and musculoskeletal disease in the elderly population. Rheumatol Int. 2001;20(3):85-87.
5. Bianchi ML, Orsini MR, Saraifoger S, Ortolani S, Radelli G, Betti S. Quality of life in post-menopausal osteoporosis. Health Qual Life Outcomes. 2005;3:78.
6. Kimberlin DK, Whitley RJ. Varicella-zoster vaccine for the prevention of herpes zoster. N Engl J Med. 2007;356(13):1338-1343.
7. Centers for Disease Control and Prevention. Arthritis-related statistics. www.cdc.gov/arthritis/data_statistics/arthritis_related_stats.htm#1. Accessed July 31, 2012.
8. AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(suppl 6):S205-224.
9. Weiner DK, Haggerty CL, Kritchvesky SB, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4(4):311-320.
10. Bosley BN, Weiner DK, Rudy TE, Granieri E. Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc. 2004;52:247-251.
11. Shega JW, Dale W, Andrews M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for pain homeostenosis? J Amer Geriatr Soc. 2012;60(1):113-117.
12. Sawyer P, Bodner EV, Ritchie CS, Allman RM. Pain and pain medication use in community-dwelling older adults. Am J Geriatr Pharmacother. 2006;4(4):316-324.
13. Won AB, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52(6):867-874.
14. Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology [published correction appears in JAMA. 1999;281(2):136]. JAMA. 1998;279(23):1877-1882.
15. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimeters? Pain. 1997;72(1-2):95-97.
16. PainKnowledge.org. Iowa Pain Thermometer Scale. www.painknowledge.org/physiciantools/Pain_Thermometer/Iowa%20Pain%20Thermometer%20Scale.pdf. Accessed July 31, 2012.
17. International Association for the Study of Pain. Faces Pain Scale–Revised.
www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/FacesPainScaleRevised/default.htm. Accessed July 31, 2012.
18. Herr K. Pain assessment strategies in older patients. J Pain. 2011;12(3):S3-S13.
19. American Medical Directors Association. Pain Assessment in Advanced Dementia (PAINAD) Scale. http://web.missouri.edu/~proste/tool/cog/painad.pdf. Accessed July 31, 2012.
20. Pain Assessment Checklist for Seniors with Limited Ability to Communicate
(PACSLAC). http://www.rgpc.ca/best/PAIN%20Best%20Practices%20-%20ML%20Vanderhorst%20(June%2007)/PACSLAC.pdf. July 31, 2012.
21. Papaleontiou M, Henderson CR Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353-1369.
22. Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324-1334.
23. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic noncancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physicians. 2008;11(suppl 2):S5-S62.
24. Buckeridge D, Huang A, Hanley J, et al. Risk of injury associated with opioid use in older adults. J Am Geriatr Soc. 2010;58(9):1664-1670.
25. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979-1986.
26. Katz KD. Tramadol is an opioid. J Med Toxicol. 2008;4(2):145.
27. Papaeleontiou M, Henderson CR, Turner BT, et al. Outcomes associated with opioid use in the treatment of chronic non-cancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353-1369.
28. Smith H. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613-624.
29. Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(suppl 2):S133-S153.
30. Matzke GR, Chan GLC, Abraham PA. Codeine dosage in renal failure [letter]. Clin Pharm. 1986;5(1):15-16.
31. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, and oxycodone). Pain Pract. 2008;8(4):287-313.
32. Quigley C, Wiffen P. A systematic review of hydromorphone in acute and chronic pain. J Pain Symptom Manage. 2003;25(2):169-178.
33. Kalso E. Oxycodone. J Pain Symptom Manage. 2005;29(suppl 5):S47-S56.
34. Heiskanen T, Matzke S, Haakana S, Gregov M, Vuori E, Kalso E. Transdermal fentanyl in cachectic cancer patients. Pain. 2009;144(1-2):218-222.
35. Lugo RA, Satterfield KL, Kern SE. Phamacokinetics of methadone. J Pain Palliat Care Pharmacother. 2005;19(4):13-24.
36. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 1998;3(3):421-430.
37. Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96(5):627-632.
38. Griessinger N, Sittl R, Likar R. Transdermal buprenorphine in clinical practice-a post-marketing surveillance study in 13,179 patients. Curr Med Res Opin. 2005;21(8):1147-1156.
39. Gupta DK, Avram MJ. Rational opioid dosing in the elderly: dose and dosing intervals when initiating opioid therapy. Clin Pharmacol Ther. 2012;91(2):339-343.
40. Rauck RL. What is the case for prescribing long-acting over short-acting opioids for patients with chronic pain? A critical review. Pain Pract. 2009;9(6):468-479.
41. Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82(1):87-96.
42. American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
43. Tumer N, Scarpace PJ, Lowenthal DT. Geriatric pharmacology: basic and clinical considerations. Annu Rev Pharmacol Toxicol. 1992;32:271-302.
44. Davies DF, Shock NW. Age changes in glomerular filtration rate, effect of venal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29(5):496-507.
45. Verbeeck RK. Pharmacokinetics and dose adjustments in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147-1161.
46. Murtagh FE, Chai MO, Donohoe P, Edmonds PM, Higginson IJ. The use of opioid analgesia in end-stage renal disease patients managed without dialysis: recommendations for practice. J Pain Palliat Care Pharmacother. 2007;21(2):5-16.
47. Pham PC, Toscano E, Pham PM, Pham PA, Pham S, Pham PT. Pain management in patients with chronic kidney disease. NDT Plus. 2009;2(2):111-118.
48. Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19(9):2542-2554.
Disclosures:
Funding for the time to write this article came from career development award K23AG029815 from the National Institute on Aging, but this agency had no role in the content, review, or approval of this article. The authors and series editor report no other relevant financial relationships.
Address correspondence to:
Joseph W. Shega, MD
Department of Geriatrics and Palliative Medicine
5841 S. Maryland Ave
Chicago, IL 60637
jshega@gmail.com
Article series summary:
This is the second article in a continuing series on pain management in older adults. Subsequent articles in this series will discuss the management of musculoskeletal pain in elders and palliative care in older adults with acute and chronic cancer pain. These articles will be published in future
issues of Clinical Geriatrics®.


