The Newly Licensed Pneumococcal Conjugate Vaccine: Questions—and Answers
Invasive pneumococcal disease (IPD) in children can cause serious illness—including meningitis, pneumonia, and bacteremia—and death. Fortunately for children, their families, and their pediatricians, the incidence of IPD in children younger than 5 years has dropped significantly following the widespread adoption of the pneumococcal conjugate vaccine (PCV).1
The initial PCV used in the United States is known as PCV7 because it includes 7 pneumococcal serotypes. Before PCV7 was licensed in 2000, these 7 serotypes accounted for about 90% of cases of IPD in US children under the age of 6 years.2 Even at that time, however, there was concern about whether other serotypes not contained in the vaccine might eventually become more common (so-called replacement serotypes). This has in fact happened, and serotypes not contained in the vaccine—especially 19A—are seen more often now.
| Table 1 — Streptococcus pneumoniae serotypes contained in the 13-valent pneumococcal conjugate vaccine | ||
| 1 3 4 5 | 6A 6B 7F 9V 14 | 18C 19A 19F 23F |
In response to these shifts, a PCV containing 13 serotypes (PCV13) (Table 1) has been developed and was licensed for use in the United States on February 24, 2010. This new vaccine includes the serotypes responsible for more than 60% of IPD cases in young US children in recent years,3 and it is anticipated that PCV13 will essentially replace PCV7.
This brief review addresses 4 important questions that are likely to arise as pediatricians make the transition to the new vaccine.
Will PCV13 induce protective antibodies against all 13 serotypes and, in turn, prevent IPD caused by these serotypes?
PCV7 has been shown to induce antibodies to the 7 serotypes it contains and also to reduce the frequency of IPD caused by these serotypes. In like manner, PCV13 has been shown to induce antibodies to the 6 additional serotypes without obvious adverse effect on the immune responses to the 7 serotypes contained in PCV7. For some serotypes, antibody concentrations measured by enzyme immunoassay have been slightly lower following immunization with PCV13 than they are after immunization with PCV7. However, functional antibody responses as measured by opsonophagocytosis assay have been comparable; thus, the significance, if any, of the slightly lower antibody responses is unclear.
For younger children who have already received 1 or more doses of PCV7, a combined schedule of PCV7 followed by PCV13 has been recommended. These combined PCV7/PCV13 schedules may result in concentrations of antibodies to the 6 additional serotypes that are lower than the concentrations that will be seen in children who receive 4 doses of PCV13 (at ages 2, 4, 6, and 12 to 15 months). The clinical significance, if any, of these lower antibody responses is unknown.
PCV13 was licensed on the basis of the serological responses observed in vaccinees and by analogy to the clinical protection afforded by PCV7 rather than on the basis of actual trials of its own clinical efficacy. Postlicensure monitoring of IPD and the causative serotypes will thus be needed to confirm the effectiveness of PCV13 in further reducing the clinical burden of IPD. The potential benefits of PCV13 compared with PCV7 in preventing noninvasive pneumococcal disease, such as otitis media, likewise remain to be demonstrated
Will PCV13 cause more adverse events than PCV7?
In one research study in which adverse events were actively solicited from the parents of vaccinees, 20% or more of infants and children who received PCV13 had local and systemic reactions.4 Local reactions included pain, redness, induration, and swelling; systemic reactions included fever, irritability, decreased appetite, and either increased or decreased sleepiness. It is important to note, however, that the frequency and severity of reactions following PCV13 were similar to those observed with PCV7. Both the vaccine manufacturer and the CDC will conduct postmarketing surveillance to detect adverse events.
PCV13 is given intramuscularly and is supplied in single-dose, prefilled syringes. The vaccine contains an aluminum phosphate adjuvant but no thimerosal or latex. PCV13 is contraindicated for persons who have had a severe allergic reaction (such as anaphylaxis) to any component of PCV13 or PCV7 or to any vaccine containing diphtheria toxoid.
The cost of PCV7 is $71.04 per dose in the public sector (CDC contract) and $83.88 per dose in the private sector. The cost of PCV13 has been estimated at $91.75 per dose in the public sector (CDC contract) and $108.75 per dose in the private sector5; thus, the new vaccine is somewhat more expensive. Because PCV13 will replace PCV7, the manufacturer (Wyeth/Pfizer) has offered to give credit for unused PCV7 from private stock.
What about children who have already received 1 or more doses of PCV7?
The recommendations for use of PCV13 depend on 2 factors:
| • | Whether a child has received any previous doses of PCV7. |
| • | Whether the child has a chronic disease or immunocompromising condition that makes him or her more susceptible to IPD. |
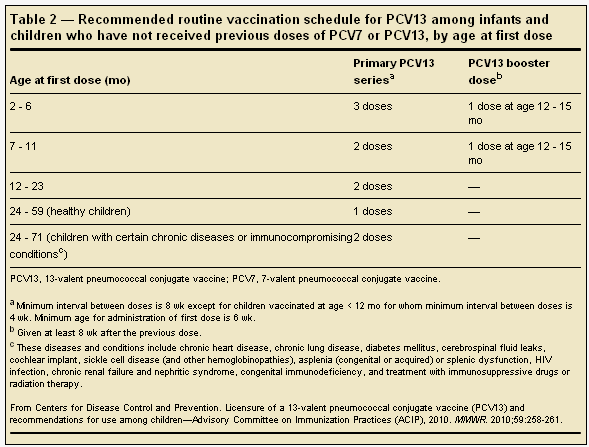
For children who have not received any previous doses of PCV7, the basic schedules for PCV13 are similar to those currently recommended for PCV7 (Table 2). For children who have received 1 or more previous doses of PCV7, the series can be completed with PCV13 (Table 3).
It is recommended that children aged 14 to 59 months who have already completed an age-appropriate schedule with PCV7 receive a single supplemental dose of PCV13. Routine use of PCV13 is not recommended for healthy children 5 years and older; however, for children with underlying conditions, the upper age limit for administration of the single supplemental dose of PCV13 is extended to 71 months. A supplemental dose of PCV13 is also recommended for children up to age 71 months who have underlying conditions and who have received the 23-valent pneumococcal polysaccharide vaccine (PPSV23). PCV13 should be given at least 8 weeks after the last dose of PCV7 or PPSV23. Finally, a single dose of PCV13 may be given to children aged 6 to 18 years who are at risk for IPD because of a chronic disease or immunocompromising condition, whether or not they have previously received PCV7 or PPSV23.
The recommendations for use of PCV13 will no doubt seem less complicated once the transition from PCV7 to PCV13 is complete. For guidance in the interim, more complete information about PCV13 and recommendations for its use will soon be available from the American Academy of Pediatrics' Red Book Committee, the CDC's Advisory Committee on Immunization Practices (ACIP), and the vaccine manufacturer. At present, the ACIP's provisional recommendations (http://www.cdc.gov/vaccines/recs/provisional/downloads/pcv13-mar-2010-508.pdf) and the FAQ section on the PCV13 page of the CDC's Web site (http://www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-hcpfaqs.htm) are helpful.
| Table 3 — Recommended transition schedule from PCV7 to PCV13 vaccination among infants and children, according to number of previous PCV7 doses received | ||||
| Infant series | Booster dose | Supplemental PCV13 dose | ||
| 2 mo | 4 mo | 6 mo | ≥ 12 moa | 14 - 59 mob |
| PCV7 | PCV13 | PCV13 | PCV13 | — |
| PCV7 | PCV7 | PCV13 | PCV13 | — |
| PCV7 | PCV7 | PCV7 | PCV13 | — |
| PCV7 | PCV7 | PCV7 | PCV7 | PCV13 |
| PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine. a No additional PCV13 doses are indicated for children aged 12 - 23 mo who have received 2 or 3 doses of PCV before age 12 mo and at least 1 dose of PCV13 at age ≥ 12 mo. b For children with underlying medical conditions, a single supplemental PCV13 dose is recommended through age 71 mo. For a list of diseases and conditions included in this recommendation, see Table 2. From Centers for Disease Control and Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. 2010;59:258-261. | ||||


