Interaction Between Simvastatin and Cranberry Juice in an Elder
Key words: Simvastatin, rhabdomyolysis, CYP3A4, cranberry, inhibitor effect.
___________________________________________________________________________
The use of dietary products and supplements for health improvement is becoming increasingly popular among all age groups. In 2007, the out-of-pocket costs of complementary and alternative medicine (CAM) products in the United States reached nearly $34 billion.1 The lack of scrutiny from the US Food and Drug Administration (FDA) has led to a scarcity of randomized controlled trials on CAM products and their concurrent use with prescription drugs. The interactions between CAM products and prescription drugs can lead to considerable morbidity. In a 2007 survey, the likelihood of such interactions was estimated to be 40%.2
We present the case of a 67-year-old woman who was admitted to the hospital because of rhabdomyolysis and hepatitis. Her presentation was consistent with exposure to a toxin or drug. The patient’s drug regimen, which included simvastatin, was stable for the previous 2 years. She informed us that she began to drink cranberry juice every day for 2 weeks prior to this hospital admission. To the best of our knowledge, this is the first clinical report of a possible interaction between cranberry juice and simvastatin.
Case Presentation
A 67-year-old white woman presented to the emergency department with severe pain, which she rated as a 10 out of 10 on a numeric pain intensity scale, and weakness in the muscles of her upper and lower extremities for the past 6 days. The patient’s medical history was significant for diabetes mellitus, moderate obesity, essential arterial hypertension, coronary artery disease, hyperlipidemia, atrial fibrillation, and mitral valve replacement with a mechanical valve. For the past 2 years, she had tolerated a stable drug regimen of insulin glargine, 20 units daily; insulin aspart, 3 units before each meal; carvedilol, 12.5 mg twice a day; aspirin, 81 mg daily; warfarin, 6 to 7 mg daily; furosemide, 40 mg daily; simvastatin, 40 mg daily; daily multivitamins; and calcium with vitamin D, 500 mg/200 IU twice a day.
On physical examination, the patient was alert and oriented, anxious, and afebrile. Her lungs were clear to auscultation, with a respiratory rate of 22 breaths per minute. An irregular heart rhythm with metallic mitral valve sounds and a heart rate of 70 to 80 beats per minute were noted. Her blood pressure was 130/60 mm Hg, her abdomen was soft and nontender on palpation, and her feet demonstrated mild edema. The muscles of her upper and lower extremities were tender and revealed a muscle strength of 4 out of 5. Deep tendon reflexes were symmetrical and preserved (2+) on both her upper and lower extremities, and there was no sensory deficit. Laboratory abnormalities included elevation of creatine kinase (16,280 U/L; normal, 40-150 U/L), alanine aminotransferase (244 U/L; normal, 10-40 U/L), and aspartate aminotransferase (703 U/L; normal, 10-30 U/L).
The patient was admitted to the hospital for the rhabdomyolysis and hepatitis. Panels for hepatitis A, B, and C were negative. Simvastatin was discontinued, as it was considered the cause of her conditions. The patient was given intravenous fluids and symptomatic treatment. The disease course was relatively benign, with the rhabdomyolysis and hepatitis resolving within 1 week and 1 month, respectively.
Discussion
Many conditions and factors can lead to rhabdomyolysis. In our patient’s case, the cause was unlikely to have an autoimmune etiology, given its benign course. In addition, its temporary association with hepatitis was consistent with exposure to a toxin or drug. Overall, simvastatin is well tolerated, but among the medications taken by the patient, it was the only one known to cause adverse hepatic and muscular events. Typically, elevation of transaminases is asymptomatic, and has been reported to occur at a rate of 0.1% to 2%.3,4 In large clinical trials and retrospective analyses, the rate of myopathy and rhabdomyolysis did not exceed 0.1%.3-6 However, our patient’s case supports the possibility of an interaction between cranberries and simvastatin, which led to her rhabdomyolysis. What follows is an overview of simvastatin, including how it is metabolized, as well as a review of cranberries, including their effects on lipid profiles, their documented interactions with various metabolic pathways, and the factors that affect their interactions with the CYP450 3A4 system and its substrates.
Simvastatin Overview
Hepatotoxicity and myotoxicity are more likely to occur with a higher dose of simvastatin7; however, organ exposure to this drug also depends on its pharmacokinetics, bioavailability, and half-life. Normally, simvastatin undergoes extensive presystemic (first-pass) metabolism, with <5% of the administered dose reaching the systemic circulation.8 Simvastatin is eliminated from the systemic circulation via metabolism in the liver and has a half-life of 1 to 3 hours.8 The drug is a substrate of the cytochrome P450 3A4 (CYP3A4) system.8,9 Cytochrome P450 enzymes are found in the small intestine and in the liver, with P450 3A4 being the most abundant, accounting for 80% of P450 enzymes in the small intestine and for 40% to 50% of P450 enzymes in the liver.10 The CYP3A4 system metabolizes >50% of xenobiotics, including pharmaceuticals.9 Although the mass of intestinal P450 3A4 enzymes is about 1% of P450 3A4 enzymes in the liver,10 contribution of the intestinal P450 3A4 enzymes to first-pass metabolism is significant and comparable with the role of hepatic P450 3A4 enzymes, a finding demonstrated in metabolic studies of midazolam11 and cyclosporine.12 Any change in CYP3A4 system activity alters simvastatin kinetics and systemic exposure. Many factors can lead to a decline in activity of the CYP3A4 system, including aging, diseases of the intestine and liver, and, most importantly, exposure to CYP3A4 inhibitors (eg, antifungals, macrolides, calcium channel blockers, amiodarone, ranolazine, antidepressants, cyclosporine, HIV-protease inhibitors, nefazodone, grapefruit juice).8,9,13
Simvastatin-type pharmacokinetics with extensive first-pass extraction is very sensitive to CYP3A4 inhibitors. Suppression of first-pass metabolism creates a “drug bubble in plasma” and a dramatic increase in systemic exposure.13 In a 1998 study by Lilja and colleagues,14 coadministration of simvastatin with grapefruit juice increased the peak concentration of simvastatin in plasma approximately 9-fold and systemic exposure to simvastatin 16-fold. Such an increase is potentially toxic. Patients who are especially vulnerable and more likely to experience myotoxicity are those older than 65 years, smokers, persons receiving a simvastatin dose of more than 40 mg daily, and individuals with hypertension, diabetes mellitus, hepatic dysfunction, electrolyte abnormalities, or hypothyroidism.4,6,15 Simultaneous use of inhibitors of the CYP3A4 system increases the relative risk of myopathy 6-fold.4,13 Cellular mechanisms of statin myotoxicity involve disruption of oxidative phosphorilation in the mytochondria, decreased synthesis of coenzyme Q, and altered membrane structure and function.7
The case patient had no changes in electrolyte levels, fluid volume status, or metabolism of glucose, protein, or lipids. Her drug regimen was stable for the past 2 years. Development of simvastatin toxicity after 2 years of treatment is unexpected and was likely caused by a change in simvastatin pharmacokinetics and organ exposure to the drug. This encouraged a search for a CYP3A4 inhibitor. When we reviewed the patient’s dietary history, we learned that for 2 weeks prior to her presentation, she began to consume 12 to 16 ounces of a diet cranberry juice drink (7% cranberry juice) per day.
Cranberries Overview and Effects on Lipid Profiles
Cranberries (Vaccinium macrocarpon) are a very popular product and an example of a functional food. Functional foods are products with scientific evidence of health benefits,16 but the FDA regulates the claims that manufacturers can make with regard to the ability of these products to diagnose, prevent, treat, or cure diseases. Unlike ordinary foods and liquids, which are sources of nutrients and calories, functional foods are designated to enhance function and reduce the risk of illness.16 Cranberries improve the defense of the urinary system by blocking the adhesion of the common uropathogens Escherichia coli and Enterococcus faecalis to the urinary tract epithelium.17 Cranberries are rich in natural antioxidants and have been used to treat atherosclerosis and to regulate lipid metabolism. In a double-blind, randomized, placebo-controlled trial, patients (mean age, 65 ± 1 year) with type 2 diabetes mellitus were given powdered cranberry extract to assess its impact on lipid profiles.18 After 12 weeks, those receiving the powdered cranberry extract showed decreases in total cholesterol (TC) levels by 7.5%, low-density lipoprotein cholesterol (LDL) levels by 12.1%, and the ratio of TC to high-density lipoprotein (HDL) cholesterol by 7%. There was no change in the content of oxidized LDL, HDL, triglycerides, C-reactive protein, and glycemic control.18 In a 2006 study of obese men (mean age, 51 years), 4 weeks of cranberry juice consumption raised HDL levels by 8.6% and decreased both the TC-to-HDL ratio and the index of oxidative stress.19
Interaction Between Cranberries and Various Metabolic Pathways
Interaction of cranberry products with CYP450 enzymes and drugs attracts a lot of interest. In 2004, the United Kingdom’s Committee on Safety of Medicines warned patients taking warfarin to avoid using cranberry products,20 based on reports of hemorrhagic complications in such patients. The presumed mechanism of hemorrhagic complications was cranberry-induced inhibition of hepatic CYP2C9, a major metabolic pathway of warfarin, and of CYP1A2, a minor metabolic pathway.21 Interaction of cranberry with the CYP2C9 system was evaluated in vitro and in vivo in a 2006 study by Greenblatt and colleagues.22 In the study, 14 healthy volunteers received a single dose of cranberry juice followed by a single dose of flurbiprofen 100 mg (a CYP2C9 probe). The cranberry juice did not alter the CYP2C9-mediated clearance of flurbiprofen, leading the authors to conclude that a pharmacokinetic interaction with warfarin is highly unlikely. Interactions between cranberry juice and the CYP1A2 system was evaluated in a 2007 study by Lilja and associates.23 In this study, cranberry juice was administered to 10 healthy volunteers for 4 days prior to administration of a drug cocktail comprised of 1 mg of tizanidine, 0.5 mg of midazolam, and 10 mg of warfarin, which are probes of CYP1A2, CYP3A4, and CYP2C9, respectively. Cranberry juice did not increase the peak plasma concentration of these probe drugs or of their metabolites, and the anticoagulant effects of warfarin were not altered, leading the authors to conclude that “a pharmacokinetic mechanism for the cranberry juice–warfarin interaction [is] unlikely.” Finally, in a 2009 prospective, randomized, double-blind study by Ansell and colleagues,24 cranberry juice caused only a minimal and insignificant increase in the international normalized ratio (INR). Therefore, neither pharmacokinetic studies,22,23 nor a pharmacodynamic clinical study,24 support a meaningful interaction between cranberry juice and warfarin. The patient presented in our case report was taking warfarin and had no hemorrhagic complications and no rise in INR.
Several pharmacokinetic studies addressed the interaction between cranberry juice and the CYP3A4 system. These studies varied in their methodology and the probe drugs used. Uesawa and Mohri25 assessed the effects of cranberry and grapefruit juices on the kinetics of nifedipine, a 3A4 substrate, in rats. Both juices suppressed first-pass metabolism and increased the bioavailability of and exposure to nifedipine by 60%, but did not affect its systemic metabolism and half-life. Ngo and colleagues26 administered three 240-mL doses of cranberry juice to 16 healthy volunteers followed by 5 mg of midazolam, another 3A4 substrate. The first-pass metabolism was inhibited, bioavailability and exposure to midazolam increased by approximately 30%, and, like with nifedipine, systemic metabolism of midazolam did not change. This study26 stands out for its preliminary in vitro testing of five cranberry juice products in enterocytes to identify a product capable of inhibiting enteric CYP3A in humans, enabling the most potent cranberry juice to be used, and its use of a higher dose of midazolam.
In a study by Grenier and colleagues,27 cranberry juice, pomelo juice, or water were administered with a single dose of cyclosporine, a 3A4 substrate, to 12 healthy male volunteers under fasting conditions, with a 14-day washout period between each dose. Cranberry juice did not change the kinetics of cyclosporine; however, this agent is also subjected to efflux transport in enterocytes by P-glycoprotein, which can affect its net bioavailability. It is possible that an increase in cyclosporine bioavailability observed with pomelo juice involved inhibition of the P-glycoprotein. In the aforementioned study by Lilja and colleagues,23 cranberry juice was administered for 4 days before a drug cocktail that included 0.5 mg of midazolam was used to probe the 3A4 system. In this study, cranberry juice did not affect the kinetics of the 0.5-mg midazolam dose or impact 3A4 activity.
Factors Affecting Interactions With the CYP450 3A4 System
Various agents can interact with the CYP450 3A4 system, including drugs, juices, and other natural products, leading to adverse reactions. These interactions are contingent on several factors, which are listed in the Table and discussed in the text that follows.
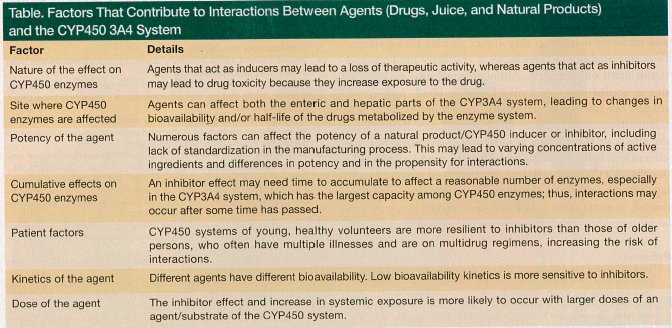
Inducers versus inhibitors. A drug, juice, or natural product can either induce or inhibit CYP450 enzymes. Inducers stimulate synthesis of new enzymes, which may lead to a loss of therapeutic activity.9,13 St. John’s wort is a well-known example of a natural inducer. Inhibitors increase exposure to a drug, which may lead to drug toxicity. Both drugs and natural products can act as reversible or irreversible enzyme inhibitors. Competitive, reversible inhibitors, such as antifungals,13 occupy enzymes for the duration of their metabolism. Irreversible inhibitors, like calcium channel blockers and erythromycin, have metabolites that form permanent chemical bonds with enzymes, a process known as mechanism-based inhibition.13,28 With exposure to irreversible inhibitors and structural damage, activity of the CYP3A4 system is restored only upon synthesis of new enzymes.13,28 Grapefruit juice works as a competitor the first few hours following administration, and it then causes irreversible structural changes in 3A4 enzymes in the small intestine.29,30 Other fruit juices have also shown potent mechanism-based inhibitory effects on the 3A4 system in vitro.31 The pharmacokinetic effect of cranberry juice was similar to grapefruit juice in the nifedipine rat study,25 and the mechanism behind this effect requires further research.
Hepatic versus enteric effects. Inhibitors can affect both the enteric and hepatic parts of the CYP3A4 system.28 Grapefruit juice, for instance, affects the enteric part only. Kupferschmidt and colleagues32 reported that grapefruit juice increased systemic exposure to oral but not to intravenous midazolam, and Lundahl and colleagues33 reported that grapefruit juice increased systemic exposure to oral but not to intravenous felodipine. Based on the results of several studies,25,26 it is likely that cranberries also affect only the enteric part of the 3A4 system.
Lack of standardization. Unlike drugs, juices and other natural products are not standardized with regard to their biological activity; thus, their activity may vary between brands and years of production. Preliminary in vitro selection of the most active cranberry juice helped to reveal the inhibitor effect of cranberries in the study by Ngo and colleagues.26
Cumulative effects. An inhibitor effect may need time to accumulate to affect a reasonable amount of enzymes, especially in the CYP3A4 system, which has the largest capacity among CYP450 enzymes. Single doses of cranberry juice may not provide adequate exposure and meaningful inhibition. A relatively low (7%) concentration of the diet cranberry juice drink consumed by our patient might explain the 2-week latency period.
Patient factors. The activity of the 3A4 system depends on an individual’s age and health status. This variability affects outcomes of studies, especially those with a small number of participants. Certainly, the CYP450 systems of young, healthy volunteers are more resilient to inhibitors than those of older persons, who often have multiple illnesses and are on multidrug regimens.
Drug kinetics and dosage. The kinetics of drugs, juices, and natural products can vary; thus, their interactions with inhibitors also vary. For instance, midazolam has a bioavailability of 40% to 50%, whereas that of simvastatin is <5%.8,13 Studies have shown grapefruit juice to increase systemic exposure to midazolam by 52%30 and to simvastatin by 1500%.14 Low bioavailability kinetics is more sensitive to inhibitors and the change is substantial.
The dose of the drug interacting with a natural product/CYP450 inhibitor can also affect the reaction. With a relatively large capacity of the CYP3A4 system, the inhibitor effect and increase in drug exposure might be seen with a large but not a small dose of a drug or natural product used, as was observed with 5-mg and 0.5-mg doses of midazolam subjected to interaction with cranberry juice.23,26
Conclusion
To the best of our knowledge, our case report is the first clinical report of a possible interaction between cranberry juice and simvastatin. Simvastatin is pharmacokinetically sensitive to inhibitors, and cranberries revealed an inhibitor effect in both animal and human studies. When an unexpected occurrence of simvastatin toxicity following long-term use occurs, a search for a change in its pharmacokinetics by a 3A4 inhibitor is warranted. Natural products should not be omitted from this search, as these products can affect the 3A4 system and suddenly change drug kinetics.
The favorable effects of cranberries on lipid metabolism and cardiovascular illness may attract patients taking statins; thus, potential interactions between cranberry products and statins merit our attention. Although conclusive evidence of this is needed from randomized, double-blind, prospective studies, our case demonstrates that natural products are bioactive substances that require pharmacovigilance. Research on the effects of these agents is warranted, particularly because interactions between a drug and a natural product, especially one that is a CYP450 inhibitor, can be turned into an advantage when a calculated augmentation of the drug’s effect is desired. In some cases, use of a natural product may prove to be more appealing than the addition of a new medication.
References
1. Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;30(18):1-14.
2. Bush TM, Rayburn KS, Holloway SW, et al. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13(2):30-35.
3. Pedersen TR, Berg K, Cook TJ, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156(18):2085-2092.
4. Cziraky MJ, Willey MJ, McKenney JM, et al. Statin safety: an assessment using an administrative claims database. Am J Cardiol. 2006;Suppl 97:61C-68C.
5. Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis
in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585-2590.
6. Gaist D, Rodriguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology. 2001;12(5):565-569.
7. Ucar M, Mjorndal T, Dahlqvist R. HMG-Coa Reductase inhibitors and myotoxicity. Drug Safety. 2000;22(6):441-457.
8. Drugs.com. Simvastatin. www.drugs.com/monograph/simvastatin.html. Accessed July 31, 2012.
9. Wilkinson GR. Drug metabolism and variability among patients in drug response.
N Engl J Med. 2005;352(21):2211-2221.
10. Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie.” Drug Metab Dispos. 2006;34(5):880-886.
11. Paine MF, Shen DD, Kunze KL, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60(1):14-24.
12. Kolars JC, Awni WD, Merion RM, et al. First-pass metabolism of cyclosporine by the gut. Lancet. 1991;338(8781):1488-1490.
13. Link B. In-vivo phenotyping of CYP3A using midazolam as probe drug: development of new approaches based on highly sensitive LS-MS/MS methods [inaugural dissertation]. Basel, Switzerland: University of Basel; 2011.
14. Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther. 1998;64(5):477-483.
15. Ronaldson KJ, O’Shea JM, Boyd IW. Risk factors for rhabdomyolysis with simvastatin and atorvastatin. Drug Safety. 2006;29(11):1061-1067.
16. Scientific concepts of functional foods in Europe. Consensus document. Br J Nutr. 1999;81(suppl 1):S1-S27
17. Sobel JD. Bacterial etiologic agents in the pathogenesis of urinary tract infection. Med Clin North Am. 1991;75(2):253-273.
18. Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med. 2008;25(12):1473-1477.
19. Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr. 2006;96(2):357-364.
20. Committee on Safety of Medicines. Interaction between warfarin and cranberry juice: new advice. Curr Prob Pharmacovigilance. 2004;30:10.
21. Rindone JP, Murphy TW. Warfarin-cranberry juice interaction resulting in profound hypoprothrombinemia and bleeding. Am J Ther. 2006;13(3):283-284.
22. Greenblatt DJ, von Moltke LL, Perloff ES, Luo Y, Harmatz JS, Zinny MA. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: in vitro and clinical studies. Clin Pharmacol Ther. 2006;79(1):125-133.
23. Lilja JJ, Backman JT, Neuvonen PJ. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam—probes of CYP2C9, CYP1A2, and CYP3A4. Clin Pharmacol Ther. 2007;81(6):833-839.
24. Ansell J, McDonough M, Zhao Y, Harmatz JS, Greenblatt DJ. The absence of an interaction between warfarin and cranberry juice: a randomized, double-blind trial. J Clin Pharmacol. 2009;49(7):824-830.
25. Uesawa Y, Mohri K. Effects of cranberry juice on nifedipine pharmacokinetics in rats. J Pharm Pharmacol. 2006;58(8):1067-1072.
26. Ngo N, Yan Z, Graf TN, et al. Identification of a cranberry juice product that inhibits enteric CYP3A-mediated first-pass metabolism in humans. Drug Metab Dispos. 2009;37(3):514-522.
27. Grenier J, Fradette C, Morelli G, Merritt GJ, Vranderick M, Ducharme MP. Pomelo juice, but not cranberry juice, affects the pharmacokinetics of cyclosporine in humans. Clin Pharmacol Ther. 2006;79(3):255-262.
28. Zhou S, Yung Chan S, Cher Goh B, et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44(3):279-304.
29. Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25(11):1228-1233.
30. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
31. Kim H, Yoon YJ, Shon JI, et al. Inhibitor effects of fruit juices on CYP3A4 activity. Drug Metab Dispos. 2006;34(4):521-523.
32. =Kupferschmidt HH, Ha HR, Ziegler WH, Meier PJ, Krähenbühl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
33. ==Lundahl J, Regårdh CG, Edgar B, Johnsson G. Effects of grapefruit juice ingestion—pharmacokinetics and haemodynamics of intravenously and orally administered felodipine in healthy men. Eur J Clin Pharmacol. 1997;52(2):139-145.
Disclosures:
The authors report no relevant financial relationship
Address correspondence to:
Gregory Goldenberg, MD
2792 Ocean Avenue, 6th Floor
Brooklyn, NY 11229
gregonline_2000@yahoo.com


