Initiating Injectable Treatment of Type 2 Diabetes: A Focus on Glucagon-Like Peptide-1 Receptor Agonists
Author:
Paul S. Jellinger, MD, MACE
Citation:
Jellinger PS. Initiating injectable treatment of type 2 diabetes: a focus on glucagon-like peptide-1 receptor agonists. Consultant. 2017;57(5):266-277.
ABSTRACT: Achievement of glycemic control is associated with reductions in diabetic complications and is an important aspect of managing type 2 diabetes. However, maintenance of glycemic control can be difficult for patients who have trouble adhering to complicated treatment regimens or who face disease progression. This review examines the literature on injectable glucagon-like peptide-1 receptor agonists (GLP-1RAs) and their potential benefits. GLP-1RAs are now recommended in treatment guidelines as either a potential first-line treatment or for treatment intensification. GLP-1RAs stimulate insulin release and suppress glucagon secretion through glucose-dependent mechanisms. This mechanism of action minimizes the risk of hypoglycemia and makes GLP-1RAs well suited for early treatment. In addition, GLP-1RAs reduce body weight and are simpler to administer than insulin. Moreover, several GLP-1RAs reduce glycemic fluctuations and have demonstrated improvements in cardiovascular outcomes and risk factors such as systolic blood pressure, lipid levels, and triglyceride levels. For some patients, GLP-1RAs as an early therapy or treatment intensification may be preferable to other oral or insulin-based regimens.
KEYWORDS: Glucagon-like peptide-1 receptor agonists, type 2 diabetes, glycemic control, glycemic fluctuations, cardiovascular outcomes
The primary treatment goals for patients with type 2 diabetes (T2D) are to achieve and maintain optimal glycemic control, reducing microvascular, and potentially macrovascular, complications.1,2 Oral glucose-lowering agents are a mainstay of diabetes management; however, many patients do not achieve adequate glycemic control with oral medications alone. Despite advances in treatment and the availability of multiple classes of agents, only approximately 52%3 to 59%4 of patients with T2D in the United States achieve glycated hemoglobin (A1C) levels below 7.0%. In a study of patients with T2D initiating treatment with metformin or a sulfonylurea (n = 3070), approximately 30% failed to achieve A1C below 7.0% after 1 year of treatment, approximately 50% failed after 2 years, and approximately 70% failed after 3 years.5 Furthermore, among patients who initially achieved A1C below 6.5% with oral treatment, approximately 40% failed to maintain A1C below 6.5% at 1 year, approximately 70% failed after 2 years, and approximately 90% failed after 3 years. Thus, the addition of other classes of medication, including injectable therapy, is often necessary to achieve treatment goals.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that stimulates insulin release following oral intake of glucose.6 The effects of incretin hormones are diminished in patients with T2D7; however, GLP-1 function is retained in T2D, and administration of GLP-1 receptor agonists (GLP-1RAs) can help restore the incretin effect in these patients.8 This review discusses challenges in the treatment of T2D and the rationale and clinical evidence for intensifying treatment using a GLP-1RA as the first injectable agent in properly selected patients.
Clinical Challenges in T2D Treatment
The treatment of T2D is complex, and challenges exist in achieving successful long-term treatment, including minimizing the risks of hypoglycemia, glycemic fluctuations, and weight gain.
Hypoglycemia, particularly nocturnal hypoglycemia, has been observed in a substantial proportion of patients, especially elderly individuals, with some experiencing severe hypoglycemic episodes.9 Symptomatic hypoglycemia of at least moderate severity has been associated with poor treatment adherence among patients with T2D receiving stable doses of metformin and a sulfonylurea.10 Among patients with T2D initially receiving metformin, with treatment intensified with sulfonylureas (n = 39,990) or insulin (n = 2948), the incidence of hypoglycemia was 24.6 events per 1000 person-years with sulfonylureas and 30.9 events per 1000 person-years with insulin.11 In addition, high rates of asymptomatic, or “silent,” hypoglycemia have been reported in studies using continuous glucose monitoring (CGM) for patients receiving metformin plus a sulfonylurea,9,12 with elderly patients at particular risk. Thus, selecting treatments for T2D that have a lower risk of hypoglycemia is an important clinical goal.
Short-term changes in blood glucose levels, or glycemic fluctuations, which include both peaks and troughs, are an emerging therapeutic target in T2D.13 In patients with T2D, basal glucose levels are elevated, and loss of postprandial control results in further elevations (excursions) after meals; thus, blood glucose levels in patients with T2D can fluctuate dramatically throughout the day despite achieving A1C targets (Figure 1).14

Ambulatory glucose profiles of (A) a participant with normal glucose tolerance and (B) a patient with type 2 diabetes. Glucose target set between 70 and 140 mg/dL (parallel horizontal lines). Shaded area indicates the area under the median curve. Panel A: Participant without type 2 diabetes who monitored glucose continuously for 30 days (A1C, 5.4%; mean glucose concentration from continuous glucose monitoring, 105 ± 19 mg/dL). Panel B: Participant with type 2 diabetes (A1C, 7.0%; mean glucose concentration from continuous glucose monitoring, 147 ± 45 mg/dL). The profile shows a higher glucose burden (shaded area), wide overnight glucose variability (interquartile range), and significant and prolonged postprandial gluose excursions (rate of change in the median curve). Reprinted from Endocrine Practice, vol 15, Mazze R, Strock E, Morgan B, Wesley D, Bergenstal R, Cuddihy R. Diurnal glucose patterns of exenatide once weekly: a 1-year study using continuous glucose monitoring with ambulatory glucose profile analysis, 326-334, copyright 2009, with permission from the American Association of Clinical Endocrinologists.101
Some evidence suggests that glycemic fluctuations may be associated with cardiovascular complications.15 The mechanisms postulated and experimentally demonstrated include acute endothelial dysfunction, increased oxidative stress, and enhancement of a proinflammatory and procoagulable state.16-19 An increased risk of cardiovascular mortality and all-cause mortality was observed among patients with T2D and impaired glucose as determined by 2-hour postprandial glucose (PPG) concentration in one DECODE study group analysis.15 The risk for cardiovascular events and death was amplified by an elevated 2-hour PPG at all levels of fasting blood glucose. Similarly, an increased risk for cardiovascular events was observed in patients with T2D with increased 2-hour PPG levels in the Framingham Offspring Study.20 Glycemic fluctuations, evaluated by mean amplitude of glycemic excursion (MAGE) and PPG excursion, were associated with the presence and severity of coronary artery disease in patients with T2D (n = 344).21 In addition, glycemic fluctuations may be associated with diabetic peripheral neuropathy, cardiovascular autonomic neuropathy, and gray-matter atrophy in the limbic and central autonomic systems.22-24 Based on these findings, controlling glycemic fluctuations is becoming a treatment goal in patients with T2D, and selecting treatments that minimize these fluctuations may prevent complications.
Another challenge for patients with T2D is that a number of treatments, including sulfonylureas, thiazolidinediones, and insulin, are associated with weight gain.25 The benefits of weight loss in patients with T2D include improved glycemic control, improved lipid profile, and reductions in blood pressure (BP).26 A meta-analysis of randomized studies among overweight or obese patients with T2D showed that a weight reduction of 5% or more appears necessary to achieve benefits in glycemic control, lipid profile, and BP27; thus, treatments that do not cause weight gain in these patients are ideal.
Treatment Approaches With GLP-1RAs
GLP-1 has a number of potentially beneficial effects in T2D, including A1C reduction, delayed gastric emptying, and body weight loss.28 The GLP-1RAs that have been approved in the United States are summarized in the accompanying Table.29 In addition to these agents, semaglutide is a long-acting GLP-1RA that is currently being evaluated in phase 3 studies.30 Treatment with GLP-1RAs has shown to reduce A1C levels and body weight, both as monotherapy and in combination with other glucose-lowering agents, in randomized, phase 3 trials.31-67
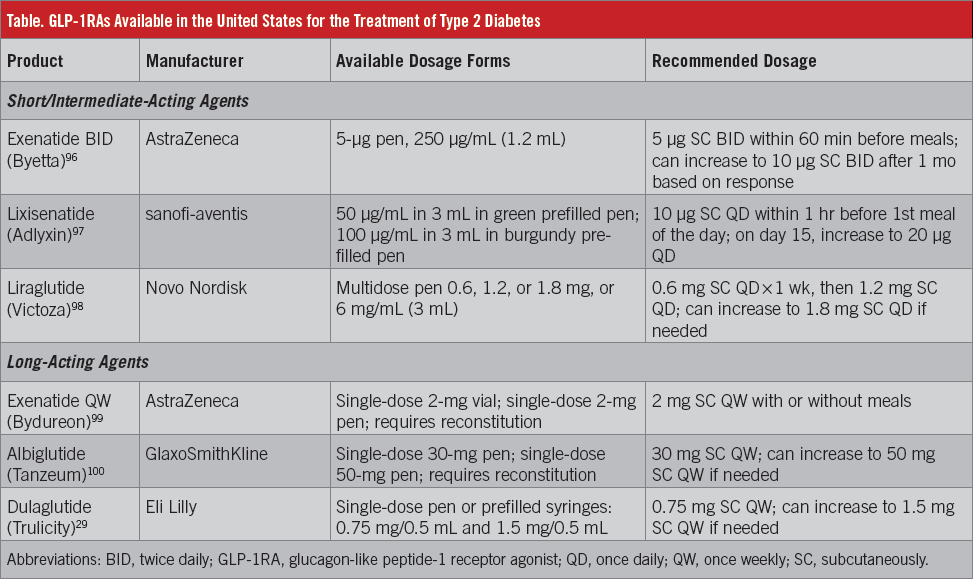
Several treatment options are available for T2D, and optimal management is individualized based on patient factors and medication characteristics.26 The American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) treatment algorithms for glycemic control encompass a stepwise approach based on A1C levels (Figure 2).26 Patients with an A1C of less than 7.5% typically begin monotherapy, with metformin as the recommended first-line treatment. GLP-1RAs are also among the agents recommended as a first-line therapy because they are associated with a low risk of hypoglycemia and with reductions in both fasting glucose and PPG. For patients with an A1C of 7.5%, dual therapy is recommended, followed by triple therapy if their goal is not met with dual therapy (Figure 2). The AACE/ACE guidelines recommend an emphasis on lifestyle modifications for all patients throughout treatment.26
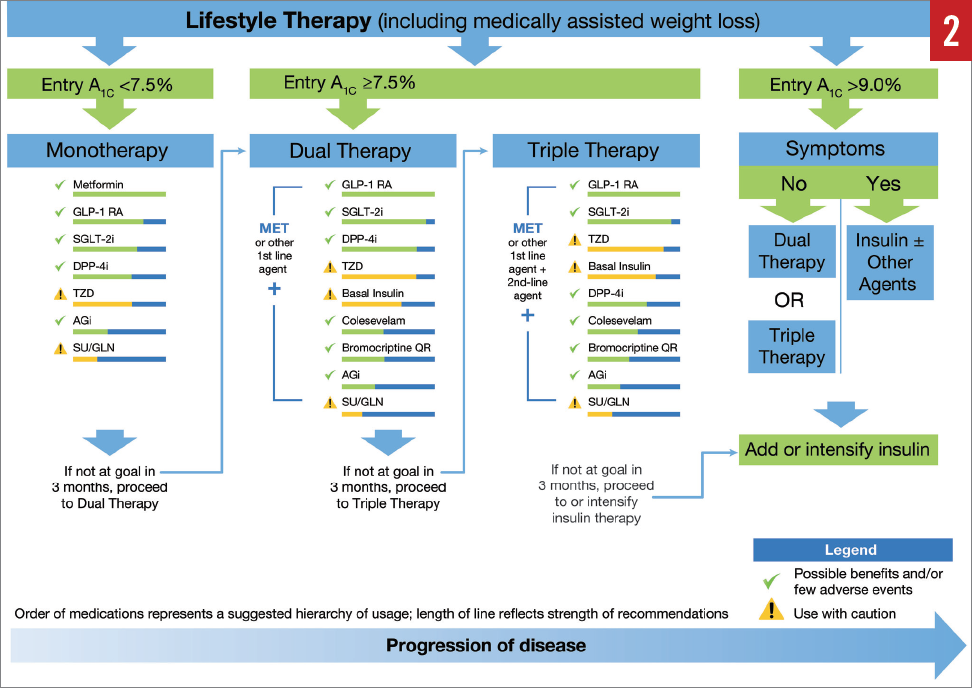
AACE/ACE glycemic control algorithm. Abbreviations: A1C, glycated hemoglobin; AGi, α-glucosidase inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLN, glinide; GLP-1RA, glucagon-like peptide-1 receptor agonist; MET, metformin; QR, quick release; SGLT-2i, sodium-glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione. Adapted with permission from the American Association of Clinical Endocrinologists ©2016 AACE. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive type 2 diabetes management algorithm 2016. Endocr Pract. 2016;22:84-113.26
Similarly, guidelines from the American Diabetes Association (ADA)2 recommend that if A1C cannot be controlled through lifestyle modifications alone, metformin monotherapy be initiated at or soon after diagnosis; however, if the patient has an A1C of 9.0% or greater, then initial dual therapy should be considered. If the A1C target is not achieved after 3 months, the patient should begin dual therapy with metformin plus another agent (Figure 3). The ADA guidelines do not list regimens in order of preference but recommend selection based on patient- and disease-related factors. Patients receiving dual therapy and triple therapy who do not achieve their A1C target should proceed to triple therapy and combination injectable therapy, respectively. The AACE/ACE and ADA guidelines both recommend early use of GLP-1RAs.

American Diabetes Association recommendations for glucose-lowering therapy in type 2 diabetes. Abbreviations: DPP-4, dipeptidyl peptidase-4; DPP-4-i, dipeptidyl peptidase-4 inhibitor; fxs, fractures; GI, gastrointestinal; GLP-1, glucagon-like peptide-1; GLP-1-RA, glucagon-like peptide-1 receptor agonist; GU, genitourinary; HbA1c, glycated hemoglobin; HF, heart failure; Hypo, hypoglycemia; SGLT2-i, sodium-glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
*Efficacy is very high, high, intermediate, and low with mean HbA1c reductions of potential of > 2%, > 1-2%, > 0.5-1%, and ≤ 0.5%, respectively. Cost is low, moderate, and high with daily cost in US$ of < $1, $1 to < $2, and ≥ $2, respectively.
†Consider initial therapy at this stage when HbA1c is ≥ 9%.
‡Consider initial therapy at this stage when blood glucose is ≥ 300–350 mg/dL and/or HbA1c ≥ 10-12%, especially if patient is symptomatic or if catabolic features (weight loss, ketosis) are present, in which case basal insulin + mealtime insulin is the preferred initial regimen.
§Usually a basal insulin (eg, neutral protamine Hagedorn, glargine, detemir, degludec).
American Diabetes Association, Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes, Diabetes Care, American Diabetes Association, 2015.2 Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
NEXT: Rationale for Early GLP-1RA Use, Effects on Weight Loss
Rationale for Early GLP-1RA Use
It is important for clinicians to monitor patients frequently and intensify treatment in those who do not achieve A1C goals.26 GLP-1RAs are suited for early use in T2D because they have a glucose-dependent mechanism of action, stimulating insulin release and suppressing glucagon secretion only when blood glucose concentrations are elevated, and therefore carry a low risk of hypoglycemia.68 Incretin-based treatments are unique in these effects and differ from sulfonylureas, which stimulate insulin release regardless of blood glucose levels. In addition to being a potential first-line treatment, GLP-1RAs are recommended by the AACE/ACE guidelines in combination with metformin as initial dual therapy for patients with an A1C of 7.5% or greater.26 For patients requiring triple therapy, GLP-1RAs can also be combined with metformin and a sodium-glucose cotransporter 2 (SGLT-2) inhibitor. Some patients are suitable for dipeptidyl peptidase-4 (DPP-4) inhibitors, another form of incretin-based therapy; however, patients requiring more robust reductions in A1C may require GLP-1RA therapy, since A1C reductions observed with DPP-4 inhibitors are generally modest and may diminish with long-term use.69
GLP-1RAs VS Insulin for Treatment Intensification
Until the past few years, insulin was typically chosen for patients with an A1C above 9.0% who required treatment intensification despite combination oral therapy. A newer approach for patients who are not symptomatic, which is supported by the AACE/ACE treatment algorithm, is to select a GLP-1RA or other incretin-based therapy in combination with metformin, or these 2 agents plus an SGLT-2 inhibitor, before initiating insulin.26 The feasibility of this approach was recently demonstrated in the DURATION-8 study, which found treatment with the GLP-1RA exenatide and the SGLT-2 inhibitor dapagliflozin to significantly reduce A1C levels compared with either treatment alone.70 Insulin therapy, especially prandial insulin (vs basal insulin), is associated with weight gain and a risk of hypoglycemia.71 The need for prandial insulin may be minimized by using GLP-1RAs and SGLT-2 inhibitors in combination (with metformin), an important potential benefit of these therapies. For patients with persistent elevated fasting blood glucose levels despite treatment with a triple combination of metformin, a GLP-1RA, and an SGLT-2 inhibitor, basal insulin (ideally administered at bedtime) may be necessary. Hypoglycemia can be minimized if basal insulin is used carefully in addition to other agents.
Key efficacy results (changes in A1C and body weight) observed in selected studies comparing GLP-1RAs with basal insulin are summarized in Figures 4A, 4B, 5A, and 5B. The potential advantages of GLP-1RAs over basal insulin include weight loss,51,63 reduced glycemic fluctuations,72-74 lower incidence of hypoglycemia,75 fewer injections and finger-stick blood glucose assessments, and potential cardiovascular benefits.

Summary of changes in A1C from selected studies of glucagon-like peptide-1 receptor agonists compared with basal insulin in patients with type 2 diabetes. Exenatide twice daily, liraglutide, and exenatide once weekly studies. CONFIDENCE102: 48-week follow-up, no background therapy. LEAD539: 26-week follow-up, metformin + glimepiride background therapy; EAGLE103: 24-week follow-up, metformin ± sulfonylurea background therapy; DURATION-351,104: 156-week follow-up, metformin ± sulfonylurea background therapy; Davies et al105: 26-week follow-up, metformin ± sulfonylurea background therapy; Inagaki et al106: 26-week follow-up, biguanide ± thiazolidinedione background therapy. Abbreviations: A1C, glycated hemoglobin; BID, twice daily; QW, once weekly.

Summary of changes in A1C from selected studies of glucagon-like peptide-1 receptor agonists compared with basal insulin in patients with T2D. Albiglutide and dulaglutide studies. HARMONY-457: 52-week follow-up, metformin ± sulfonylurea background therapy; HARMONY-659: 26-week follow-up, metformin ± pioglitazone or α-glucosidase inhibitor; AWARD-263: 52-week follow-up, metformin + glimepiride background therapy; AWARD-464: 26-week follow-up, ± metformin background therapy. Abbreviation: A1C, glycated hemoglobin.

Summary of changes in body weight from selected studies of glucagon-like peptide-1 receptor agonists compared with basal insulin in patients with type 2 diabetes. Exenatide twice daily, liraglutide, and exenatide once weekly studies. CONFIDENCE102: 48-week follow-up, no background therapy; LEAD539: 26-week follow-up, metformin + glimepiride background therapy; EAGLE103: 24-week follow-up, metformin ± sulfonylurea background therapy; DURATION-351,104: 156-week follow-up, metformin ± sulfonylurea background therapy; Davies et al105: 26-week follow-up, metformin ± sulfonylurea background therapy; Inagaki et al106: 26-week follow-up, biguanide ± thiazolidinedione background therapy. Abbreviations: BID, twice daily; QW, once weekly.

Summary of changes in body weight from selected studies of glucagon-like peptide-1 receptor agonists compared with basal insulin in patients with type 2 diabetes. Albiglutide and dulaglutide studies. HARMONY-457: 52-week follow-up, metformin ± sulfonylurea background therapy; HARMONY-659: 26-week follow-up, metformin ± pioglitazone or α-glucosidase inhibitor; AWARD-263: 52-week follow-up, metformin + glimepiride background therapy; AWARD-464: 26-week follow-up, ± metformin background therapy.
Effects on weight. GLP-1RAs delay gastric emptying and enhance satiety, thereby reducing food intake and potentially resulting in weight loss. In the DURATION-3 study of exenatide once weekly (QW) added to metformin with or without a sulfonylurea, exenatide-treated patients had a mean weight reduction of 2.6 kg over 26 weeks compared with a weight gain of 1.4 kg in patients receiving add-on basal insulin.51 In a 2011 meta-analysis of 21 randomized, clinical trials evaluating GLP-1RA use among patients with T2D or obesity (N = 6411), dose-dependent mean reductions in body weight ranged from 0.2 to 7.2 kg with GLP-1RAs vs a mean reduction of 2.9 kg in the control group.76 A subgroup analysis showed that greater weight loss was observed with higher doses of GLP-1RAs.
Effects on glycemic fluctuations. Initial evidence for reduced glycemic fluctuations with GLP-1RAs was found in a small pilot study of patients with T2D. Exenatide twice daily (BID) (n = 6) significantly reduced mean daily glucose levels and MAGE compared with glimepiride (n = 6) after 16 weeks.74 More recently, a prospective placebo-controlled study evaluated the quality of glycemic control with exenatide QW in patients with T2D not achieving adequate control with metformin (N = 116).72 After 10 weeks, treatment with exenatide QW plus metformin significantly reduced 24-hour least-squares mean ± standard error glucose compared with placebo plus metformin (–30.8 ± 4.5 vs –3.1 ± 4.9 mg/dL; difference: –27.8 ± 6.7 mg/dL; P < .001) and significantly reduced MAGE (difference: –18.0 ± 5.1 mg/dL; P < .001) and standard deviation (SD) of blood glucose concentrations (–6.3 ± 1.3 vs +0.7 ± 1.4 mg/dL; difference: –7.0 ± 1.8 mg/dL; P < .001). In addition, exenatide QW significantly reduced the proportion of time patients spent in the hyperglycemic range (> 180 mg/dL; exenatide QW vs placebo: –12.0% ± 3.4%; P < .001) and increased time in the target glycemic range of 70.3 to 180.0 mg/dL (exenatide QW vs placebo: +20.1% ± 4.3%; P < .001), with no increased time spent in the hypoglycemic range (nominal P = .18).
One study compared exenatide BID with rapid-acting insulin as prandial add-on therapy to insulin glargine plus metformin in patients with T2D (n = 102).73 Similar A1C levels were observed in the exenatide BID (7.1%) and rapid-acting insulin groups (7.2%) at week 26. However, exenatide BID resulted in a reduction in coefficients of variation of glucose from baseline to 26 weeks (using CGM) of –2.4 vs a slight increase (+0.4) with rapid-acting insulin (P = .047).
Dulaglutide and liraglutide have also been shown to reduce glycemic fluctuations in patients with T2D. In a subgroup analysis of 144 patients in the AWARD-4 study, patients received either dulaglutide QW (1.5 or 0.75 mg) or insulin glargine, both added to prandial insulin lispro, for 52 weeks.77 At 26 weeks, dulaglutide 1.5 mg plus prandial insulin significantly reduced glycemic fluctuations vs insulin glargine plus prandial insulin, as measured by within-patient SD (−12.6 vs −7.2 mg/dL; P < .05) and mean of daily differences (−7.2 vs +1.8 mg/dL; P < .05). Glycemic fluctuation data were similar in all treatment groups at 52 weeks. A 26-week subgroup analysis of the DUAL-1 study evaluated IDegLira (a fixed-ratio combination of insulin degludec and liraglutide) in 260 patients with T2D.78 A significantly greater reduction in mean ± SD PPG increment on a standardized meal test was observed with IDegLira vs insulin (–15.7 ± 29.7 mg/dL vs −3.1 ± 35.7 mg/dL, respectively; P = .023), with similar reductions achieved with liraglutide alone (–14.1 ± 29.2 mg/dL). A study in obese patients with T2D requiring at least 100 units of insulin per day (n = 37) who received liraglutide plus intensive high-dose insulin or standard insulin alone demonstrated a 22.5% reduction in within-day SD of mean daily glucose with liraglutide plus insulin, measured by CGM (P < .0001), with no significant change observed with standard insulin therapy alone.79
Risk of hypoglycemia. Because of their glucose-dependent mechanism of action, GLP-1RAs generally have a low risk of hypoglycemia if not combined with sulfonylureas or insulin. A meta-analysis of randomized, controlled trials of GLP-1RAs vs placebo or traditional glucose-lowering agents found that all GLP-1RAs (except dulaglutide) had a lower risk of hypoglycemia compared with insulin, and all GLP-1RAs had a lower risk of hypoglycemia compared with sulfonylureas.75
Convenience. Postprandial insulin therapy requires multiple daily injections to maintain adequate glucose control. In addition, multiple daily finger-stick capillary blood glucose assessments are required to monitor blood glucose levels and adjust the dosage to ensure adequate glucose control and avoid hypoglycemia. Treatment with a GLP-1RA allows for fewer overall injections and requires less-intense blood glucose monitoring once a target dosage is established.
Effects on cardiovascular outcomes. GLP-1RAs also have potential cardiovascular benefits for patients with T2D,80 as evidenced by their positive effects on systolic BP, triglycerides, lipids, and body weight.81-83 In a retrospective analysis of patients with T2D receiving exenatide (N = 39,275) or other glucose-lowering treatments (N = 381,218), patients who received exenatide had a reduced risk of a cardiovascular disease (CVD) event (first occurrence of myocardial infarction [MI], ischemic stroke, or coronary revascularization procedure) vs other therapies (hazard ratio [HR], 0.81; 95% CI, 0.68-0.95; P = .01), with lower rates of CVD-related hospitalization (HR, 0.88; 95% CI, 0.79-0.98; P = .02) and all-cause hospitalization (HR, 0.94; 95% CI, 0.91-0.97; P < .001).84
The large, randomized, phase 3B LEADER study evaluated cardiovascular outcomes with the short-acting GLP-1RA liraglutide among patients with T2D at high risk for CVD who were either treatment-naïve or were receiving oral glucose-lowering agents, insulin, or a combination.81 At a median follow-up of 3.8 years, significantly fewer patients receiving liraglutide had the primary composite outcome of a first occurrence of death from cardiovascular causes, nonfatal MI, or nonfatal stroke vs placebo (13.0% vs 14.9% of patients; P = .01 for superiority).
The randomized, phase 3 SUSTAIN-6 study evaluated long-term cardiovascular outcomes in patients with T2D.85 Patients aged 50 years or older with established CVD or chronic kidney disease and 1 or more cardiovascular risk factors (N = 3297) received subcutaneous semaglutide 0.5 or 1 mg QW or volume-matched placebo for 104 weeks. At a median follow-up of 2.1 years, the composite end point of first cardiovascular death, nonfatal MI, or nonfatal stroke occurred in 6.6% of patients receiving semaglutide vs 8.9% of patients receiving placebo (P = .02 for superiority), representing a 26% lower risk with semaglutide treatment.
The randomized, double-blind, placebo-controlled ELIXA study evaluated the cardiovascular effects of lixisenatide in patients with T2D (N = 6068) who had an MI or who were hospitalized for unstable angina in the previous 180 days.86 At a median follow-up of 25 months, no significant differences in cardiovascular events were observed between lixisenatide and placebo; the primary composite end point (cardiovascular death, MI, stroke, or hospitalization for unstable angina) occurred in 13.4% of patients receiving lixisenatide vs 13.2% of patients receiving placebo (P = .81 for superiority). Thus, results with GLP-1RAs to date show either an improvement or no adverse effects in cardiovascular outcomes. Several ongoing studies are evaluating cardiovascular outcomes with GLP-1RAs in patients with T2D, including HARMONY Outcomes for albiglutide87 and the EXSCEL trial for exenatide QW.88 The ongoing AWARD-10 study of dulaglutide includes cardiovascular events as a secondary end point.89
Cardiovascular outcomes studies are providing important information to aid clinical practice, with recent reports about another newer drug class, the SGLT-2 inhibitors, as well as previous reports with insulin. In the EMPA-REG OUTCOME study, the SGLT-2 inhibitor empagliflozin improved the rate of the composite outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke relative to placebo.90 Further research is warranted to evaluate potential added benefit for SGLT-2 inhibitors combined with GLP-1RAs. In some studies, evaluating the effects of insulin therapy on cardiovascular outcomes among patients with T2D has suggested increased insulin-associated CVD risk. However, clinical trials have been complicated by the presence of comorbid conditions, the use of other glucose-lowering treatments such as sulfonylureas (long considered to potentially increase CVD risk), and variability in insulin regimens. Although studies have evaluated cardiovascular effects of tight glucose control and have attempted to determine the effects of insulin on cardiovascular outcomes among patients with T2D, the presence of multiple confounding factors has made it difficult to adequately isolate the effects of insulin, thus results are conflicting.91-93
Conclusions
Measures to manage T2D are only effective if patients are adherent to their prescribed treatment regimen.94 Factors affecting treatment adherence include complexity of the treatment regimen, route of administration, adverse effects, and cost.94 Fear of insulin and fear of injection have been associated with poor outcomes in patients with diabetes.95 Use of long-acting GLP-1RAs can potentially reduce the number of injections needed to manage T2D. Effective patient education and communication between patients and clinicians regarding the potential benefits of treatment adherence is important in improving outcomes.
GLP-1RAs are a valuable therapy for T2D and are recommended as early injectable treatment. The AACE/ACE 2016 algorithm recommends GLP-1RAs be added to metformin if A1C goal is not achieved by metformin alone after 3 months, and to be combined with metformin for initial combination therapy when A1C is 7.6% to 9.0%. Emerging evidence has demonstrated positive cardiovascular outcomes within the GLP-1RA class, and additional trials are under way. Given the benefits on glycemic profiles and weight management, early use of injectable agents such as GLP-1RAs is a commonsense approach to treatment with a strong basis in evidence. Compelling evidence exists to support GLP-1RAs as the first injectable therapy for patients who do not achieve adequate glucose control with oral glucose-lowering medications.
Paul S. Jellinger, MD, MACE, is an endocrinologist at The Center for Diabetes and Endocrine Care in Fort Lauderdale, Florida, and a professor of clinical medicine at the University of Miami Miller School of Medicine in Miami, Florida.
Acknowledgment: Amy Zannikos, PharmD, on behalf of inScience Communications, Springer Healthcare, in Philadelphia, Pennsylvania, provided medical writing support funded by AstraZeneca.
Funding/Support: The development of this manuscript was supported by AstraZeneca.
Disclosures: Dr Jellinger has served on the speakers bureau for AstraZeneca, Boehringer Ingelheim, Merck, and Novo Nordisk.
REFERENCES:
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149.
- Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;369(3):287-288.
- Wong ND, Patao C, Wong K, Malik S, Franklin SS, Iloeje U. Trends in control of cardiovascular risk factors among US adults with type 2 diabetes from 1999 to 2010: comparison by prevalent cardiovascular disease status. Diab Vasc Dis Res. 2013;10(6):505-513.
- Cook MN, Girman CJ, Stein PP, Alexander CM. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med. 2007;24(4):350-358.
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
- Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46-52.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705.
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in type 2 diabetes—more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol. 2015;9(5):999-1005.
- Walz L, Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wändell P. Impact of symptomatic hypoglycemia on medication adherence, patient satisfaction with treatment, and glycemic control in patients with type 2 diabetes. Patient Prefer Adherence. 2014;8:593-601.
- Roumie CL, Min JY, Greevy RA, et al. Risk of hypoglycemia following intensification of metformin treatment with insulin versus sulfonylurea. CMAJ. 2016;188(6):E104-E112.
- Simonyi G, Csitári G, Gasparics R, et al. Silent hypoglycaemic episodes among well controlled type 2 diabetic patients treated with sulfonylureas [abstract 962]. Diabetologia. 2015;58(suppl 1):S465.
- Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: an emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013;102(2):86-95.
- Kohnert K-D, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J Diabetes. 2015;6(1):17-29.
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397-405.
- Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681-1687.
- Zhang X-G, Zhang Y-Q, Zhao D-K, et al. Relationship between blood glucose fluctuation and macrovascular endothelial dysfunction in type 2 diabetic patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 2014;18(23):3593-3600.
- Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1.
- Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015;120:28-39.
- Meigs JB, Nathan DM, D’Agostino RB Sr, Wilson PWF. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25(10):1845-1850.
- Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19.
- Xu F, Zhao L-h, Su J-b, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139.
- Jun JE, Jin S-M, Baek J, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70.
- Cui X, Abduljalil A, Manor BD, Peng C-K, Novak V. Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS One. 2014;9(1):e86284.
- Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf. 2007;30(12):1127-1142.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84-113.
- Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447-1463.
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824-830.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Co; 2015.
- Nauck MA, Petrie JR, Sesti G, et al; Study 1821 Investigators. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39(2):231-241.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092-1100.
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD; Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628-2635.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083-1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146(7):477-485.
- Marre M, Shaw J, Brändle M, et al; LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268-278.
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84-90.
- Garber A, Henry R, Ratner R, et al; LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481.
- Zinman B, Gerich J, Buse JB, et al; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32(7):1224-1230.
- Russell-Jones D, Vaag A, Schmitz O, et al; Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055.
- Buse JB, Rosenstock J, Sesti G, et al; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47.
- Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE; EFC6018 GetGoal-Mono Study Investigators. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012;35(6):1225-1231.
- Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013;36(9):2543-2550.
- Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013;36(9):2497-2503.
- Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab. 2013;15(11):1000-1007.
- Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36(9):2489-2496.
- Rosenstock J, Raccah D, Korányi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36(10):2945-2951.
- Bolli GB, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2014;31(2):176-184.
- Russell-Jones D, Cuddihy RM, Hanefeld M, et al; DURATION-4 Study Group. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252-258.
- Drucker DJ, Buse JB, Taylor K, et al; DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240-1250.
- Bergenstal RM, Wysham C, MacConell L, et al; DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431-439.
- Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375(9733):2234-2243.
- Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301-1310.
- Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381(9861):117-124.
- Reinhardt R, Nauck MA, Stewart M, et al. HARMONY 2 results at week 52 primary endpoint: once-weekly albiglutide monotherapy for patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Presented at: 49th Annual Meeting of the European Association for the Study of Diabetes; September 23-27, 2013; Barcelona, Spain. Abstract 903.
- Reusch J, Stewart MW, Perkins CM, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab. 2014;16(12):1257-1264.
- Ahrén B, Johnson SL, Stewart M, et al; HARMONY 3 Study Group. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141-2148.
- Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484.
- Home PD, Shamanna P, Stewart M, et al. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab. 2015;17(2):179-187.
- Rosenstock J, Fonseca VA, Gross JL, et al; Harmony 6 Study Group. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-2325.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289-297.
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168-2176.
- Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159-2167.
- Giorgino F, Benroubi M, Sun J-H, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241-2249.
- Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057-2066.
- Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149-2158.
- Dungan KM, Tofé Povedano S, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349-1357.
- Sorli C, Harashima S-i, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251-260.
- Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care. 2011;34(suppl 2):S264-S271.
- Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D. Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of long-term randomised controlled trials. BMJ Open. 2014;4(6):e005442.
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004-1016.
- Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011;34(2):510-517.
- Frías JP, Nakhle S, Ruggles JA, et al. Exenatide once weekly improved 24-hour glucose control and reduced glycaemic variability in metformin-treated participants with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(1):40-48.
- FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016;39(6):973-981.
- Irace C, Fiorentino R, Carallo C, Scavelli F, Gnasso A. Exenatide improves glycemic variability assessed by continuous glucose monitoring in subjects with type 2 diabetes. Diabetes Technol Ther. 2011;13(12):1261-1263.
- Li Z, Zhang Y, Quan X, et al. Efficacy and acceptability of glycemic control of glucagon-like peptide-1 receptor agonists among type 2 diabetes: a systematic review and network meta-analysis. PLoS One. 2016;11(5):e0154206.
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771.
- Jendle J, Testa MA, Martin S, Jiang H, Milicevic Z. Continuous glucose monitoring in patients with type 2 diabetes treated with glucagon-like peptide-1 receptor agonist dulaglutide in combination with prandial insulin lispro: an AWARD-4 substudy. Diabetes Obes Metab. 2016;18(10):999-1005.
- Holst JJ, Buse JB, Rodbard HW, et al. IDegLira improves both fasting and postprandial glucose control as demonstrated using continuous glucose monitoring and a standardized meal test. J Diabetes Sci Technol. 2015;10(2):389-397.
- Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
- Saraiva FK, Sposito AC. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol. 2014;13:142.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
- Simó R, Guerci B, Schernthaner G, et al. Long-term changes in cardiovascular risk markers during administration of exenatide twice daily or glimepiride: results from the European exenatide study. Cardiovasc Diabetol. 2015;14:116.
- Sun F, Wu S, Wang J, et al. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2015;37(1):225-241.
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90-95.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247-2257.
- Effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in subjects with type 2 diabetes mellitus. NCT02465515. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02465515. Accessed April 12, 2017.
- Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
- A study of dulaglutide (LY2189265) in participants with type 2 diabetes mellitus (AWARD-10). NCT02597049. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02597049. Accessed April 12, 2017.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
- Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98(2):668-677.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
- ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319-328.
- García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175-194.
- Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25(6):1413-1420.
- Byetta [package insert]. Wilmington, DE: AstraZeneca; 2015.
- Adlyxin [package insert]. Bridgewater, NJ: sanofi-aventis; 2016.
- Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
- Bydureon [package insert]. Wilmington, DE: AstraZeneca; 2015.
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2015.
- Mazze R, Strock E, Morgan B, Wesley D, Bergenstal R, Cuddihy R. Diurnal glucose patterns of exenatide once weekly: a 1-year study using continuous glucose monitoring with ambulatory glucose profile analysis. Endocr Pract. 2009;15(4):326-334.
- Xu W, Bi Y, Sun Z, et al. Comparison of the effects on glycaemic control and β-cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel-group trial (the CONFIDENCE study). J Intern Med. 2015;277(1):137-150.
- D’Alessio D, Häring H-U, Charbonnel B, et al; EAGLE Investigators. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170-178.
- Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464-473.
- Davies M, Heller S, Sreenan S, et al. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368-1376.
- Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther. 2012;34(9):1892-1908.


