Chest Pain: Does This Patient Have Cardiac Ischemia?
ABSTRACT: The first step in the evaluation of acute chest pain is to determine whether the symptoms are cardiac in origin. If you suspect possible myocardial ischemia, ask the patient about risk factors for coronary artery disease. Focus the physical examination on signs of heart disease, especially heart failure. The ECG is key in the immediate evaluation of chest pain. ST-segment elevation of more than 1 mm in two contiguous leads, or more than 1 mm ST-segment depression moves cardiac ischemia to the top of the list of differential diagnoses. The next step is to define the patient’s immediate risk of adverse outcomes to determine the urgency of the workup and treatment plan. Troponin measurement is the most sensitive and specific test for the diagnosis of myocardial infarction. If the diagnosis remains uncertain, consider ordering a transthoracic echocardiogram.
__________________________________________________________________________________________________________________________________________________
Acute chest pain accounts for many presentations and subsequent admissions to the hospital each year. It is one of the more anxiety-provoking presentations to evaluate, both because the stakes are high and the determination of an exact cause is often difficult.
What follows is meant to help guide you through a thorough evaluation of chest pain and rule out myocardial ischemia, the most serious cause, quickly and efficiently. It should also help you risk-stratify patients and decide on a focused diagnostic evaluation and treatment plan.
DETERMINING THE ETIOLOGY OF THE SYMPTOM
First, make an assessment of whether a patient’s chest pain is likely cardiac in origin or not. Sometimes a patient’s description of the pain and the risk factor profile for the existence of coronary artery disease (CAD) make that determination fairly easy. Commonly, however, this is not the case, and we must broaden our differential diagnosis.
To do so, we have built a framework for ourselves that you may find useful. We try to simplify the otherwise broad differential diagnosis by grouping the numerous potential causes of chest pain anatomically and mechanistically.
 Ischemic causes of chest pain. For example, classic angina results from chronic narrowing of the epicardial coronary arteries due to atherosclerosis. We start there and then move on to other disease processes that cause chest pain by the same ischemic mechanism: ie, decreased blood flow to the epicardial coronary arteries. These are:
Ischemic causes of chest pain. For example, classic angina results from chronic narrowing of the epicardial coronary arteries due to atherosclerosis. We start there and then move on to other disease processes that cause chest pain by the same ischemic mechanism: ie, decreased blood flow to the epicardial coronary arteries. These are:
• Thrombotic occlusion (classic myocardial infarction [MI]).
• Vasospasm (Prinzmetal’s angina, idiopathic, cocaine-induced).
• Aortic valve stenosis.
• Coronary artery embolism.
• Aortic dissection with extension into coronary ostium.
• Primary coronary artery dissection.
Next, we consider diseases that cause ischemia at the level of the microvasculature (endocardium), as opposed to the epicardium. These include:
• Hypertension.
• Tachycardia (due to an acute disease such as pneumonia and anemia; or atrial fibrillation, flutter, or atrial tachycardia with rapid ventricular rate).
• Dilated cardiomyopathy.
• Syndrome X (chest pain in the presence of normal coronary arteries on angiography, thought to be due to microvascular ischemia, endothelial cell dysfunction, and/or heightened perception of pain in the setting of any afferent stimulation. Often, nonspecific ECG abnormalities will be found. Exercise stress tests are abnormal. Syndrome X may account for 20% of patients with chest pain and normal coronary arteries. The prognosis is benign with respect to mortality).1
• Tako-tsubo cardiomyopathy (stress-induced acute, reversible cardiomyopathy that presents like MI but the coronary arteries are normal).
• Inflammatory disease of the coronary arteries (coronary arteritis).
Non-ischemic causes of chest pain. Once we have considered the potential culprits of chest pain caused by myocardial ischemia, we turn our attention to non-ischemic causes. Here, we transition from mechanism to anatomy, and group the diseases by organ system (Table 1).
CLUES IN THE HISTORY, PHYSICAL EXAMINATION, AND ECG
History. Now comes the challenge of deciding which of these processes the patient in question actually has. Chest pain from myocardial ischemia, whether acute or chronic, often has certain features that distinguish it from non-ischemic causes. Patients most frequently describe a pressure-like pain or discomfort over the left precordium that lasts from 5 to 20 minutes and which may recur in a “stuttering”-like pattern. They may complain of a burning, a fullness, or a squeezing sensation over the precordium.
If the pain began during exertion, it is expected to resolve within minutes of cessation of that activity. Alternatively, sublingual nitroglycerin may relieve the symptoms. Patients frequently have associated symptoms such as dyspnea, fatigue, or dizziness. The pain may radiate to the arms, shoulders, jaw, or even teeth. Depending on the mechanism, the pain may start acutely. Conversely, it may occur predictably in concert with activities that the patient can identify, such as climbing stairs or walking up hills. Pain that is constant over days or occurs as episodes of sharp lancinating pain lasting seconds is rarely ischemic in origin. Pleuritic and positional qualities would be very atypical for pain associated with myocardial ischemia.
Regardless of the exact nature of a particular patient’s symptoms, if we now suspect possible myocardial ischemia, we turn our attention to developing an initial, or pre-test, probability that the patient has CAD. We review the risk factors for CAD and ask the patient specifically about modifiable risk factors. Doing so helps us determine the direction and urgency of our diagnostic and treatment plan, even before laying hands on the patient.
The modifiable risk factors are:
• Tobacco smoking.
• Diabetes.
• Hypertension.
• Hyperlipidemia.
Non-modifiable risk factors are:
• Family history (first-degree male relative with CAD diagnosed before the age of 55, or a first-degree female relative diagnosed before the age of 65).
• Age older than 55 years for men, 65 years for women.
• Personal history of CAD.
The more risk factors, the more concerned we become. By this time, even without a physical examination, it is appropriate to obtain a 12-lead ECG and basic laboratory data, which might include cardiac enzymes, depending on the nature and acuity of the clinical presentation.
Physical examination. Here, we focus on signs of heart disease, especially heart failure. Hypotension and pulmonary rales are the most concerning physical findings. Elevated jugular venous pressure or an S3 gallop may also indicate heart failure. The apical holosystolic murmur of mitral regurgitation could represent acute ischemia or chordal rupture. Patients with the latter will likely also have pulmonary edema because of acute elevation in left atrial pressures. More nonspecific findings of ischemia include pallor, diaphoresis, anxiety, and tachycardia.
ECG. While the history and physical examination are extremely helpful in establishing a diagnosis, the ECG trumps other findings in the immediate evaluation of chest pain. We try to obtain it early in the evaluation (within 10 minutes if the patient presents to the emergency department [ED]).
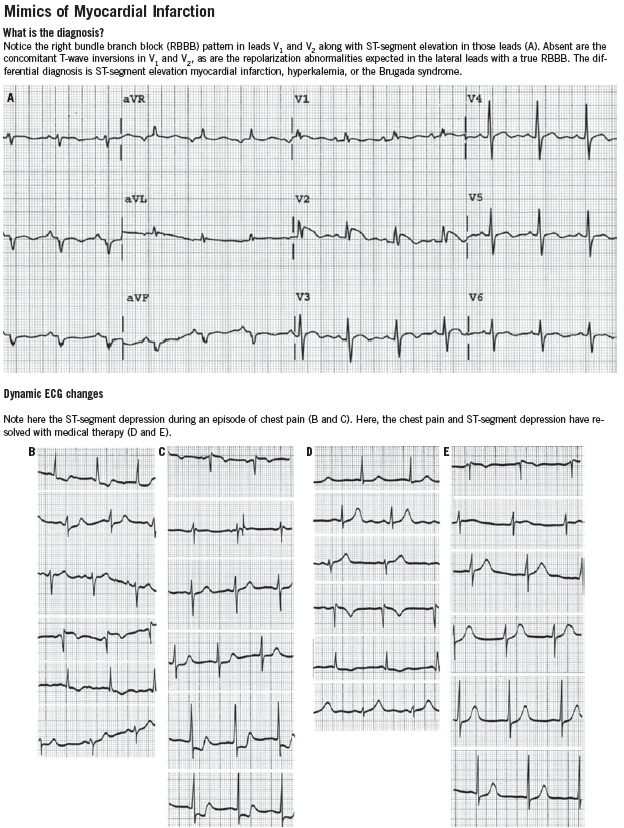
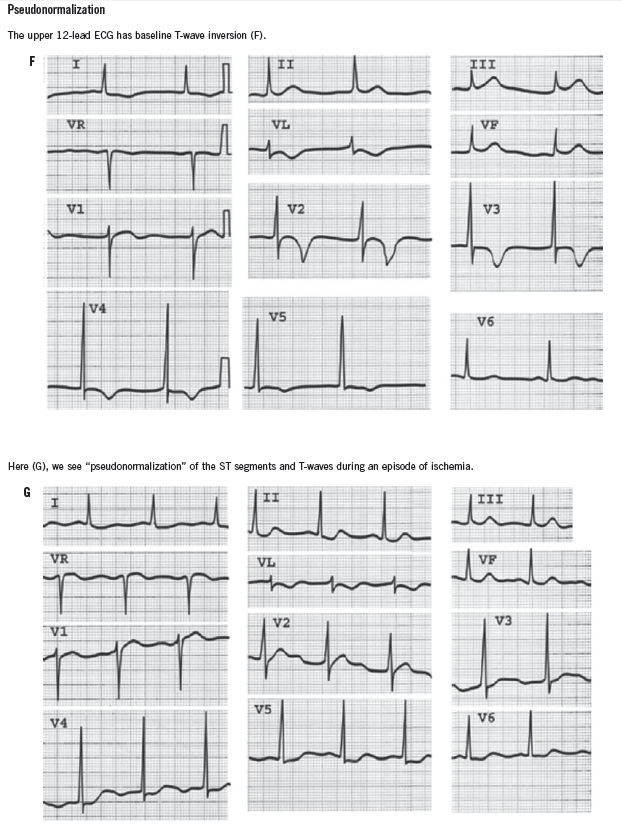
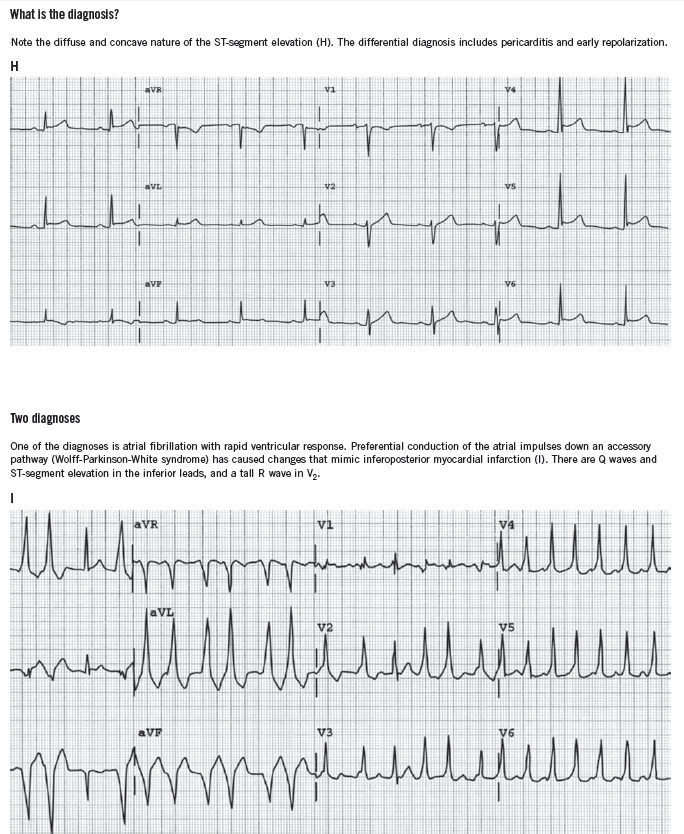
ST-segment elevation of more than 1 mm in two contiguous leads, or more than 1 mm ST-segment depression moves cardiac ischemia to the top of the list of differential diagnoses. Left bundle branch block has been considered to be an ST-segment elevation equivalent (although this is becoming controversial), unless it is known to be old, in which case the history becomes the most important determinant of the diagnostic workup. T-wave flattening and T-wave inversions are less specific for ischemia but may point to that diagnosis in the right clinical setting. Transient normalization of previously abnormal ST-T waves that resolves with the resolution of pain (“pseudonormalization”) also leads to a diagnosis of cardiac ischemia. Increased R-wave amplitude in leads V1 and V2 in the right clinical setting may indicate ischemia, past or present. Q waves in anatomically contiguous leads may occur transiently if the ischemia is transmural, or be an indicator of previous MI. If old, their presence increases the pre-test probability of CAD even in the absence of other acute ECG abnormalities.
One caveat to remember is that ST-segment depression and T-wave abnormalities in the setting of tachyarrhythmias are common. While these findings do imply myocardial ischemia, they often resolve with interventions that treat the cause of the tachyarrhythmia. We always repeat a 12-lead ECG in patients with tachyarrhythmias once the rate is better controlled or normal rhythm is restored. If the ECG abnormalities resolve with treatment of the physiologic trigger, we are reassured and classify the patient differently.
It is also important to be aware of conditions that mimic MI, such as hyperkalemia, pericarditis, myocarditis, Wolff-Parkinson-White syndrome, and early repolarization variants. ECGs that demonstrate some of these conditions can be found in the Box.
All patients should have repeat ECGs performed as their clinical course develops. Sometimes, a chest radiograph is helpful for inpatients or patients in the ED. We do not routinely use them in the outpatient setting.
These are the basics. Next, we have to define the patient’s diagnosis and risk of adverse outcomes to determine the urgency of the workup and treatment plan.
ACUTE CORONARY SYNDROMES
Assuming now that the pre-test probability for CAD is high, we need to define the patient’s immediate risk. To do so, we must decide whether or not the patient has an acute coronary syndrome (ACS). More specifically, we categorize a patient as having stable angina or ACS. This classification is important, as it defines risk of MI, death, and need for revascularization. Acute coronary syndromes encompass:
• Unstable angina (UA).
• Non–ST-segment elevation MI (NSTEMI).
• ST-segment elevation MI (STEMI).
Stable angina is chest pain (or the equivalent in a given patient, such as dyspnea, shoulder pain, etc) that occurs with exertion. A predictable and reproducible amount of activity triggers the symptom. The patient may also have associated symptoms such as dyspnea. Often he or she will describe the pain as having a pressure- or squeezing-like quality. It may radiate to the left arm, neck, or jaw. Nitroglycerin and/or the cessation of the activity associated with the pain should relieve the symptom within minutes.
In contrast, unstable angina occurs at rest, with less activity, or with greater frequency than the patient’s stable symptoms. Qualitatively, it is similar to stable angina. It may last longer than stable angina, and usually resolves but recurs. Conversely, chest pain in patients with NSTEMI or STEMI is often sudden in onset and unremitting. These patients are more likely to have concomitant dyspnea, anxiety, diaphoresis, unstable vital signs, or signs of heart failure.
The differences in presentation and risk exist because an ACS is caused by unstable plaque (or, in the case of STEMI, plaque rupture) obstructing a coronary artery, whereas stable angina is caused by chronic narrowing of the coronary arteries from atherosclerotic disease. Thus, the immediate risk, urgency of workup, and treatment are also different.
RADIOLOGIC AND LABORATORY ASSESSMENT
With the disease entities defined, we assess the chest radiograph and laboratory findings to better categorize the patient. Once you suspect an ACS, interpretation of the chest film is easy. Either there is pulmonary edema or there is not. Laboratory analysis is somewhat less straightforward.
Troponin measurement is the most sensitive and specific test for the diagnosis of MI.2-4 Levels usually peak at 24 to 48 hours after MI, and the test remains positive for up to 2 weeks after the event. On the other hand, it has a low sensitivity in the very early phase of MI, and results will be normal in many patients who present within 6 hours. In the setting of MI, it is almost always at least minimally elevated by 12 hours.
The MB fraction of creatine kinase (CK-MB) has a lower sensitivity for MI.5 Its two advantages over troponin are that it tends to peak earlier (within 12 to 24 hours) and to dissipate faster. Thus, we can use it to track the resolution of infarction after medical or procedural reperfusion. It is less specific, however, especially in the setting of skeletal muscle disease or injury, and it must be interpreted with the clinical picture in mind. We have seen countless patients in consultation for an elevated CK and CK-MB who do not have chest pain. These tests are rarely helpful when elevated in that setting. Many laboratories have discontinued the routine performance of this test.

DEFINING A PATIENT’S RISK
With this structure established, we move to quickly define the diagnosis, the risk, and the treatment. Simply put, patients with accelerating or new chest pain thought to be cardiac in nature who have negative cardiac enzyme tests have UA until proven otherwise. Those with similar symptoms, elevated cardiac enzyme levels, and no ST-segment elevation have a NSTEMI. We treat UA as a NSTEMI until we see at least two sets of negative enzyme tests separated by at least 6 to 8 hours. Obviously, those with ST-segment elevation in the setting of chest pain and its accompanying symptoms have a STEMI.
We further define high-risk patients, using retrospective and validated prediction rules from data collected during the Thrombolysis in Myocardial Infarction (TIMI) trials. From these trials the so-called TIMI risk assessment and subsequent TIMI Risk Score arose.6,7 Applying it to patients with UA, NSTEMI, and STEMI is relatively simple, and very helpful.
Unstable angina and NSTEMI. The TIMI Risk Score6 for UA/NSTEMI predicts a 14-day risk of cardiac events, namely MI, urgent revascularization, or death. Patients accrue points for various findings in their history, ECG, and cardiac enzyme measurements. It should be applied only to patients with chest pain, as that is the population in whom it was validated. The score calculation and interpretation are shown in Table 2.
STEMI. A TIMI Risk Score that predicts mortality in patients with STEMI is shown in Table 3.7

SHOULD THE PATIENT BE ADMITTED?
It is at this time that we decide on the disposition. We admit all patients with STEMI, NSTEMI, or ST-segment depression of more than 0.5 mm in more than one ECG lead for medical, interventional, or surgical treatment.
Accelerating chest pain during presentation, a TIMI Risk Score of 2 or higher, ventricular ectopy, Q waves in a specific coronary artery distribution, and new T-wave inversion convince us that the patient should be admitted for serial troponin determinations and medical therapy until MI is ruled out. If the diagnosis remains uncertain, we order a transthoracic echocardiogram (TTE) to look for ventricular wall motion abnormalities, left ventricular systolic and diastolic dysfunction, or mitral regurgitation that could suggest myocardial ischemia, infarction, or other structural heart disease. Any of these TTE findings would convince us to admit the patient and evaluate for MI. We also use a TTE to assess the extent of myocardium involved in an MI to push us toward early interventional or medical therapy. At this time, patients usually fit into one of the four risk groups listed in Table 4.8

Essentially, low-risk patients can be managed by further risk stratification (to make sure they actually are at low risk) and stress testing depending on their pre-test probability of CAD.10 Higher-risk patients should be considered for early invasive treatment (percutaneous intervention) along with heparin and platelet glycoprotein IIb/IIIa inhibitors, or more conservative medical treatment as the clinical course develops.11
In conclusion, our approach asks a few simple questions from which an expeditious diagnostic and management strategy can be decided on:
1. Is this chest pain cardiac in origin?
2. Is cardiac ischemia from obstruction or narrowing of the coronary arteries the mechanism?
3. Is the risk factor profile for CAD increasing the pre-test probability of finding CAD?
4. Is the physical examination presenting us with signs of cardiac disease, either acute or chronic, that increase our index of suspicion?
5. Is there ST-segment elevation or depression on the 12-lead ECG?
6. Is there other (albeit less specific) evidence of cardiac ischemia on the ECG?
7. Would a resting transthoracic echocardiogram help me make a clinical decision here?
8. Should I begin immediate in-hospital medical therapy, as I pursue a more in-depth workup for ACS?
REFERENCES:
1.Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005. Chapter 50.
2.Ohman EM, Armstrong PW, Christenson RH, et al, for the GUSTO IIA Investigators. Cardiac troponin T levels for risk stratification in acute myocardial ischemia. N Engl J Med. 1996;335:1333-1341.
3.Ravkilde J, Horder M, Gerhardt W, et al. Diagnostic performance and prognostic value of serum troponin T in suspected acute myocardial infarction. Scand J Clin Lab Invest. 1993;53:677-685.
4.Stubbs P, Collinson P, Moseley D, Greenwood T, Noble M. Prospective study of the role of cardiac troponin T in patients admitted with unstable angina.BMJ. 1996;313:262-264.
5.Puleo PR, Meyer D,Wathen C, et al. Use of a rapid assay of subforms of creatine kinase-MB to diagnose or rule out acute myocardial infarction. N Engl J Med. 1994;331:561-566.
6.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835-842.
7. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous TPA for treatment of infarcting myocardium early II trial sub-study. Circulation. 2000;102:2031-2037.
8. Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). 2002. Accessed July 11, 2013.
9.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
10.Lee TH, Cook EF,Weisberg M, Sargent RK,Wilson C, Goldman L. Acute chest pain in the emergency room: identification and examination of low-risk patients. Arch Intern Med. 1985;145:65-69.
11.Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISMPLUS) Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non–Q wave myocardial infarction [erratum appears in N Engl J Med1998;339:415]. N Engl J Med. 1998;338:1488-1497.


