Beyond Basal Insulin: How Glucagon-Like Peptide-1 Receptor Agonists Fit in the Spectrum of Therapeutic Options
ABSTRACT: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have proven to be effective glucose-lowering agents for the treatment of type 2 diabetes mellitus, comparable or superior to many classes of antihyperglycemic agents in reducing postprandial and fasting blood glucose levels. Furthermore, they have the added benefits of potential weight loss and minimal hypoglycemia risk. GLP-1 RAs have been shown to be safe and effective when used in combination with a range of diabetes medications, and treatment guidelines include GLP-1 RAs as a therapeutic choice at any point in the treatment algorithm. This article will discuss potential clinical strategies for adding a GLP-1 RA to basal insulin, using demonstrative case studies to illustrate circumstances in which a patient may benefit from this approach.
Although the 2 fundamental defects in type 2 diabetes mellitus (T2DM) have been traditionally viewed as insulin resistance and pancreatic beta-cell insufficiency, derangements of alpha-cell signaling and a depressed incretin effect are now understood to be crucial aspects of the disease process.1
Recognition of the importance of these abnormalities has led to the introduction of drugs focused on the incretin system, including glucagon-like peptide-1 receptor agonists (GLP-1 RAs) that act by mimicking the effects of endogenous GLP-1. This results in 4 fundamental effects typical of the GLP class:-2
•Improved satiety through a central nervous system mechanism
•Stimulation of insulin production, but only when glucose levels are elevated (hence the label “glucose-dependent insulin secretion”)
•Blunting of glucagon, but only when glucose levels are elevated, thereby preserving appropriate glucagon responses to hypoglycemia (hence the label “glucose-dependent glucagon inhibition”)
•Modulation of gastric emptying, resulting in decreased entry of caloric content into the small intestine per unit time
The potency of GLP-1 RAs for glucose reduction is comparable or superior to most other classes of antihyperglycemic agents, and favorably impacts both postprandial blood glucose and fasting glucose.3 Clinical trials have shown that GLP-1 RAs are both safe and effective when used as monotherapy,4-10 or in combination with metformin,10-39 sulfonylureas,10,14,15,18,19,21,22,24-26,28,29,32,38-41, thiazolidinediones,10,16,22,27-29,33,39,42 and insulin therapy.10,34,43-46
As longterm control of T2DM is rarely attainable with monotherapy, combination therapy is likely to be required in most patients. The most recent American Association of Clinical Endocrinologists and joint American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) guidelines on T2DM include GLP-1 RAs as a rational therapeutic choice at any point in the therapeutic algorithm, including as a first-choice combination agent with metformin and, in line with accumulating evidence of glycemic benefits, in combination with basal insulins.47,48 Here we discuss the clinical potential of using GLP-1 RAs as an add-on to basal insulin and provide case study examples of the circumstances in which a patient may benefit from this treatment strategy.
Incretin System as a Treatment Pathway
The incretin pathway forms a key part of glucose homeostasis.49 Two endogenous incretins—glucose-dependent insulinotropic peptide (GIP) and GLP-1—are peptide hormones secreted predominantly by the K cells (small intestine) and L cells (small intestine/bowel), respectively, in response to food intake (Figure).49 Currently, there are no available pharmacotherapeutic agents whose primary mechanism of action is to modulate GIP. Instead, let’s focus on GLP-1-active agents.
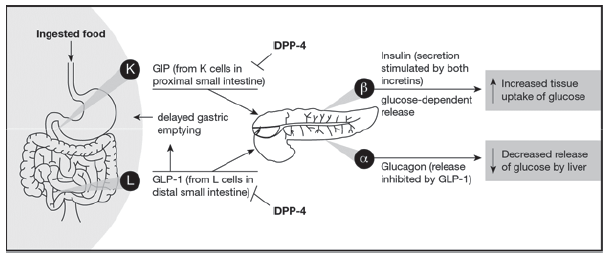
Figure. The role of GIP, GLP-1, and DPP-4 in the incretin system. Reproduced from Ross and Ekoé.49
Abbreviations: DPP-4, dipeptidyl peptidase-4; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide-1.
Under normal physiological conditions, GLP-1 increases insulin release from pancreatic beta cells in a glucose-dependent fashion. That is, in contrast to the sulfonylureas which problematically continue to stimulate insulin production even in the face of hypoglycemia, GLP-1 RAs stimulate insulin production from the beta cells only when glucose levels are elevated. In addition, GLP-1 suppresses glucagon release from the pancreatic alpha cells.49,50 This, in turn, suppresses inappropriate hepatic gluconeogenesis and glycogenolysis.
Curiously, it has been shown that glucagon levels are inappropriately high in the fasting state, which leads to fasting hyperglycemia, and—contraphysiologically—rise in the postprandial state, when glucagon levels are instead supposed to be suppressed. Glucagon suppression is also glucose-dependent; the appropriate glucagon response to hypoglycemia that restores euglycemia through enhanced glucagon-stimulated hepatic gluconeogenesis is not suppressed. The activities of native GLP-1 are limited by the activity of dipeptidyl peptidase-4 (DPP-4), which rapidly breaks down the active forms of GLP-1 in circulation resulting in a plasma half-life of only a few minutes.49
The GLP-1 response is impaired in patients with T2DM. Postprandial GLP-1 levels, expressed as both the total area under the curve (AUC) and the incremental AUC following ingestion of a set, mixed meal are significantly decreased in patients with T2DM compared with individuals with normal glucose tolerance.51 Incretin-based therapy aims to raise GLP-1 levels either by injection of synthetic GLP-1 or by oral administration of DPP-4 inhibitors, which increase incretin levels by reducing the otherwise very prompt inactivation of native GLP-1 by DPP-4.
There are currently 5 GLP-1 RAs approved by the FDA, listed here in alphabetical order: albigutide (once-weekly), dulaglutide (once-weekly), exenatide (twice-daily and once-weekly); and liraglutide (once-daily). Lixisenatide (once-daily) is licensed by the European Medicines Agency (EMA), and pending FDA approval at the time of publication.
Synthetic GLP-1 RAs are resistant to degradation by DPP-4, allowing them to remain active far longer than native GLP-1. Although oral DPP-4 inhibitors increase endogenous GLP-1 plasma levels 2- to 3-fold, GLP-1 RAs can achieve plasma levels that are 5- to 7-fold higher than physiological levels of GLP-1, leading to greater stimulation of the GLP-1 receptor.52,53 This is reflected in the significantly greater improvements in hemoglobin A1c (HbA1c) reported in head-to-head trials of GLP-1 RAs and DPP-4 inhibitors.53 Overall, GLP-1 RAs have been shown to be superior to DPP-4 inhibitors in terms of glycemic control, resulting in greater HbA1c reductions, 24-hour glucose, and 2-hour postprandial glucose reductions.54 Similarly, the additional potency of parenteral GLP-1 RAs is associated with weight loss, in contrast to DPP-4 inhibitors that are generally weight neutral.48,54,55 Further potential advantages of GLP-1 RAs compared with DPP-4 inhibitors are their beneficial effects on blood pressure and lipid profiles.56
DPP-4 inhibitors are advantageous in that they result in less mild-to-moderate GI side effects than GLP-1 RAs; they are also taken orally rather than injected. However, increased treatment efficacy with GLP-1 RAs together with greater weight loss appears to result in improved patient satisfaction compared to DPP-4 inhibitors.54
The Role of Gastric Emptying in Glucose Homeostatis
Gastric emptying is a major determinant of postprandial glycemia in healthy individuals and patients with diabetes.57 One of the less recognized pathophysiologic changes occurring early in T2DM is increased gastric emptying, which leads to too-high a caloric load for the sluggish pancreas to keep up with, and which is primarily manifested as postprandial hyperglycemia.57 In patients with T2DM not managed with insulin, delayed gastric emptying—such as that induced by GLP-1 RAs—is associated with reduced postprandial glycemic excursions.57 Hence, pharmacologically-delayed gastric emptying might be better viewed as a therapeutic (or beneficial) effect of GLP-1 RA rather than a toxic effect.
There is the suggestion that not all GLP-1 RAs share the same capacity to favorably impact postprandial glucose with long-term use.58 This may be related to the fact that the slowing of gastric emptying induced by long-acting GLP-1 RAs (eg, exenatide weekly, liraglutide daily) appears to diminish with time in contrast to short-acting agonists (eg, exenatide twice-daily, lixisenatide daily).57
Combining Basal Insulin and GLP-1 RAs: Case Studies
Several randomized clinical trials have demonstrated the benefits of combining GLP-1 RAs with basal insulin.43-45,59 Current guidelines state that the effectiveness of this combination has been illustrated over the last 3 years, with most studies demonstrating equivalent or slightly superior efficacy compared with the addition of prandial insulin, with the added benefit of weight loss and less hypoglycemia.47 Patient-centered care is the keystone of therapy for patients with T2DM and therefore, the decision to adopt a particular strategy is highly dependent on a patient’s needs and preferences. A selection of illustrative case studies is presented to demonstrate some of the potential benefits of GLP-1 RAs in combination with basal insulins.
Case 1: Your patient is not meeting HbA1c goals on basal insulin alone.
Patient 1 is a 48-year-old female who was diagnosed 7 years ago with T2DM. Her current regimen includes insulin detemir (45 U once daily) and metformin (1000 mg twice daily), as well as lisinopril (20 mg daily) and atorvastatin (20 mg daily). Recent blood tests came back normal for lipid levels, and renal and liver function, but she had an HbA1c level of 7.9%. Her fasting blood glucose was 126 mg/dL. ADA Clinical Practice Recommendations60 suggest that an HbA1c goal of <7.0% or even <6.5% would be appropriate because she fits into a group of “young age, recent onset T2DM.” (Expert opinion: evidence level for this goal was a C).
Regardless of whether her HbA1c goal is <6.5% or <7.0%, she is not at target. In her most recent physical, the patient was found to have mild retinopathy, but normal blood pressure (115/78 mm Hg) and no signs of cardiovascular disease. However, she had been gaining weight at a rate of 1.4 kg (3 lb) annually for the past 3 years, and currently weighs 78 kg (172 lb) with a body mass index (BMI) of 28.7 kg/m2.
She has not had any problematic episodes of hypoglycemia. Although she makes an effort to exercise regularly and control her diet, she reports periods of hunger particularly at night and finds herself unable to resist the occasional high-calorie binge.
Treatment
Although still relatively young with potentially many years of living ahead, the age at which Patient 1 developed T2DM is of concern for ongoing B-cell function and consequently, her ability to reach glycemic goals. Although her fasting glucose was within the ADA Clinical Practice Recommendation target, an HbA1c of 7.9%, her young age, and early retinopathy suggests further intervention with aggressive treatment may be appropriate. Although prandial insulin or another oral antidiabetes drug also can be prescribed, escalation of insulin or addition of a sulfonylurea or thiazolidinedione may lead to further weight gain.
Research
Several randomized clinical trials have reported the benefits of basal insulin in combination with a GLP-1 RA.43-45,59 Adding a GLP-1 RA to established basal insulin has been associated with significant weight loss compared with placebo.43,44,59
A recent systematic review has suggested that adding a GLP-1 RA to basal insulin is associated with additional HbA1c lowering, lower basal insulin requirements, decreased postprandial glucose levels, and weight loss, as compared to placebo.61 An analysis of individual patient data from 5 randomized clinical trials assessing the efficacy and safety of basal insulin plus insulin glulisine (n=3) or basal insulin plus lixisenatide (n=2) found that patients receiving add-on lixisenatide were more than twice as likely to meet HbA1c goals compared with those receiving basal insulin plus insulin glulisine.62 Recent data comparing addition of albiglutide (once-weekly) or prandial lispro (3 times daily) to insulin glargine illustrated comparable reductions in HbA1c levels, with weight loss and lower hypoglycemia risk in subjects treated with albiglutide.46
(Case 2 on next page)
Case 2: Your patient is experiencing hypoglycemia following the addition of basal insulin to metformin and a sulfonylurea.
Patient 2 is a 52-year-old male who was diagnosed with T2DM 8 years prior. He recently added insulin glargine (50 u once daily) to his existing antidiabetes regimen of combination metformin (1000 mg twice daily) and glimepiride (2 mg daily). In the 3 months since, he has experienced intermittent episodes of hypoglycemia. This included 1 symptomatic episode, occurring at 4 pm, during which he recorded a blood glucose level of 38 mg/dL. As a result, the patient wants to discontinue the basal insulin from his regimen.
At the current visit, he has an HbA1c of 7.6%, weight of 93.5 kg, and BMI of 30.5 kg/m2. Estimated glomerular filtration rate (GFR) is 70 mL/min with no evidence of albuminuria and blood lipids are in the normal range. Vital signs are within normal limits and the patient’s hypertension appears to be adequately managed on his current antihypertensive regimen of amlodipine.
Treatment
Sulfonylurea treatment is known to fail over time.63 In addition, many experts believe that the combination of sulfonylurea with insulin is not physiologically sensible as both work through insulin augmentation.64 Rather than discontinuing basal insulin in this case, it may be appropriate to discontinue or markedly reduce the sulfonylurea dose. Assuming the sulfonylurea was discontinued rather than reduced, we would anticipate a rise in glucose.
At this point, it would be reasonable to add a GLP-1 RA. As the response to GLP-1 RA is variable, it may be wise to at least transiently reduce the dose of insulin by 20%. If the combination is insufficient to provide glycemic control, basal insulin can be further titrated. As GLP-1 RA-induced insulin production only occurs when glucose levels are elevated, the intrinsic risk for hypoglycemia associated with these agents when used as monotherapy is inherently low.65
Research
In recent randomized clinical trials,43,45 the incidence of symptomatic hypoglycemia has been shown to increase significantly in patients who received a GLP-1 RA plus basal insulin compared with placebo. However, in the study conducted by Seino and colleagues, much of this hypoglycemia was suggested to be related to background treatment with a sulfonylurea because the incidence of hypoglycemia in patients not receiving concomitant sulfonylurea was similar to the rate observed with placebo.45
In a study conducted by Riddle and colleagues, a higher rate of symptomatic hypoglycemia with lixisenatide versus placebo was predominantly observed during the initial 6 weeks of treatment, which included the 2-step dosage increase for lixisenatide.43 Additionally, a recent systematic review concluded that the use of GLP-1 RAs as add-on to basal insulin in T2DM was associated with additional lowering of HbA1c without risk for severe hypoglycemia.61 The addition of GLP-1 RAs to insulin can be associated with a reduction in insulin dose requirements.66
Another study noted a reduction in mean daily insulin dose from 91.1 U to 52.2 U over a mean follow-up period of 7 months (where exenatide or liraglutide were added to different insulin regimens),67 resulting in a lower likelihood of insulin-related weight gain, a smaller volume of insulin injection, and reduced expense of insulin (both due to the lower dose).
Case 3: Your patient is struggling with the practicalities of rapid-acting insulin (RAI) following its addition to basal insulin and is unhappy with weight gain.
Patient 3 is a retired 69-year-old male, former smoker, with a 5-year history of T2DM. His diabetes is well-controlled (HbA1c of 6.7%) following addition of prandial RAI insulin to basal insulin (35 U basal insulin once daily plus 4 U RAI insulin before each meal) and metformin (1000 mg twice daily). Patient 3 leads an active life and struggles to manage the practicalities of administering regular RAI injections.
Since starting the RAI insulin 3 months before, his weight has increased by 2.7 kg (5.9 lb) despite an established exercise routine and maintenance of an appropriate diet. Laboratory tests reveal that his fasting glucose is slightly elevated at 132 mg/dL, but other blood chemistry and creatinine results are in the normal range. His current weight is 77 kg and he has a BMI of 25.7 kg/m2. Although only marginally overweight, the patient is unhappy with his recent weight gain and wants to lose 3 to 4 kg to take him back to his retirement weight. In addition, he wants a simplified antidiabetes regimen, which doesn’t require him to inject insulin during the daytime and at preset intervals around mealtimes, as he likes to be active and often does not follow set routines.
Treatment
The weight-lowering effects of a GLP-1 RA would be beneficial for Patient 3 and would provide an equivalent glucose-lowering effect as his current prandial insulin regimen. In an analysis of 7 randomized clinical trials evaluating liraglutide versus active comparators, patients receiving the GLP-1 RAs tested (liraglutide and exenatide) had the highest rates of weight loss (>5%), compared with the DPP-4 inhibitor sitagliptin, sulfonylurea, insulin glargine, or thiazolidinedione.68 Weight loss is attributed to the GLP-1 RA directly (through improved satiety) and indirectly to the reduction in insulin dose once GLP-1 RA treatment is initiated.
Research
A systematic review of randomized clinical trials evaluating insulin/DPP-4 inhibitors or insulin/GLP-1 RA regimens identified 15 placebo-controlled or active-comparator studies reporting outcomes of such regimens in T2DM.69 In 6 trials, the addition of DPP-4 inhibitors to insulin was associated with modest HbA1c lowering and weight neutrality. In 5 clinical trials of GLP-1 RAs added to basal insulin, HbA1c lowering was seen accompanied by reductions in weight.
Case 4: Your patient is aware of many therapeutic options, but is also concerned about cardiovascular adverse effects of diabetes treatments.
Patient 4 is a 59-year-old male with T2DM diagnosed 15 years prior. In addition, he has atrial fibrillation, peripheral vascular disease, and mild chronic kidney disease (stage 3). His weight is 128 kg (282 lb), with a waist circumference of 124 cm (49 in), and a BMI of 38 kg/m2. He is currently taking basal insulin (60 U once daily), metformin (1000 mg twice daily), enalapril (10 mg twice daily), digoxin (0.25 mg daily), metolazone (2.5 mg twice daily), furosemide (120 mg daily), and allopurinol (300 mg daily). His fasting blood glucose level was 212 mg/dL. A low-density lipoprotein cholesterol test result was 162 mg/dL, and an electrocardiogram revealed atrial fibrillation with a ventricular rate of 76 beats per min. Estimated GFR was 53 mL/min.
Patient 4 indicated his concern about cardiovascular risk and although he acknowledged the need for better glycemic control, he was worried about hypoglycemia with insulin intensification, based upon information he read about adverse cardiovascular outcomes in the Action to Control Cardiovascular Risk in Diabetes trial.70 He had read about GLP-1 RAs in an online patient group forum, had health insurance, and was keen to find out if this would be a good approach for him, particularly as he was having little success managing his weight.
Treatment
Current guidelines of the ADA recommend individualization of therapy for each patient based on their current health, comorbid conditions, hypoglycemic tolerance, and other factors. Where possible, lowering HbA1c to <7% is recommended to reduce the incidence of microvascular disease.60 This HbA1c target is appropriate for this patient because he has existing cardiovascular risk factors, but is still relatively young and may, therefore, warrant a more aggressive approach to manage this risk. There is some evidence supporting beneficial effects of GLP-1 RA on cardiovascular risk factors such as blood pressure, weight, and total cholesterol concentrations.71 Large randomized clinical studies are currently in progress to evaluate long-term effects of GLP-1 RAs on cardiovascular outcomes.72-75
Practical Considerations
There are many things to consider and evaluate when helping patients achieve their diabetes goals. The current joint 2015 ADA/EASD algorithm for treatment intensification indicates 5 commonplace considerations to address when advancing therapy: cost, efficacy, hypoglycemia risk, other adverse effects, and weight.47 Treatment intensification for patients already receiving basal insulin should focus upon whether failure to attain HbA1c goals is due primarily to excess fasting excursions, postprandial excursions, or both and selecting treatments, which specifically address these deficits.
Both fasting and postprandial hyperglycemia are important to overall glycemic control; guidelines give target ranges for both. Even though a reduction of fasting plasma glucose with basal insulin might result in the achievement of HbA1c goals, management of postprandial and fasting glucose levels may be required to maintain target HbA1c levels over time.47,76 In the case of GLP-1 RAs, the choice of most appropriate agent depends, at least in part, on the glycemic disturbance that is in most need of correction.77
For example, when patients have markedly elevated HbA1c, most of the excess glucose excursion is contributed by fasting hyperglycemia; as HbA1c levels approach target goals, postprandial hyperglycemia is the greater contributor.78 Therefore, for patients with postprandial hyperglycemia, shorter-acting GLP-1 RAs (eg, exenatide twice daily, lixisenatide daily) that have a greater effect on postprandial glucose levels largely though slowed gastric emptying may be more appropriate for patients early in the course of their disease or those nearing glycemic goals, especially where weight loss would be beneficial.79,80
Conversely, fasting hyperglycemia is more important in less well-controlled patients and therefore, long-acting GLP-1 RAs (eg, albiglutide weekly, dulaglutide weekly, exenatide weekly, liraglutide daily) that have a greater effect on fasting glucose levels through enhanced insulin secretion and decreased glucagon secretion may be more appropriate where fasting glucose levels are the primary concern.79
Coadministration of other glucose-lowering agents with insulin often requires revision of insulin dosing to minimize risk of hypoglycemia. Although guidance on this topic in the literature is limited, the authors recommend that when adding high efficacy agents to insulin (eg, GLP-1 RAs, thiazolidinediones, sodium-glucose cotransporter-2 inhibitors), a considerable reduction (20%) in basal insulin should occur to minimize risk of hypoglycemia. Insulin doses can be retitrated if insufficient glucose control occurs as a result of reduced insulin.
Concerns have previously been raised regarding incretin-based drugs and an increased risk of pancreatitis and pancreatic cancer, which were extensively investigated by the FDA and EMA.81-83 The FDA and EMA recently published the results of their investigations, with both agencies agreeing that, “…assertions concerning a causal association between incretin-based drugs and pancreatitis or pancreatic cancer, as expressed recently in the scientific literature and in the media, are inconsistent with the current data.”84
Although the agencies explain that they have not reached a final conclusion regarding such a causal relationship, and that both agencies continue to investigate possible safety signals, they note that the current knowledge is reflected adequately in the information or labeling for such products.83
A joint statement from the ADA, EASD, and the International Diabetes Federation points out that GLP-1 RAs and DPP-4 inhibitors have been extensively studied in regulatory and clinical trials and have demonstrated at least equivalence, if not superiority, over many other diabetes medications without safety concerns.81 For incretin-based therapy, the conclusions of the regulatory authorities suggest that the risk of pancreatitis or pancreatic cancer is currently unproven; therefore, recommendations in treatment guidelines regarding the suitability and potential benefits of GLP-1 RAs stand.
GLP-1 RA therapy is an established option in the management of glycemia in T2DM and primary care providers should feel confident in prescribing these agents across the spectrum of disease. Control of both fasting and postprandial hyperglycemia in patients with T2DM is crucial to mimicking the tight overall glycemic control seen in normal individuals. To this end, the combination of a GLP-1 RA with basal insulin represents a rational choice in the management of both forms of hyperglycemia. Although basal insulin acts to control fasting hyperglycemia, GLP-1 RAs also improve postprandial glucose excursions in addition to reducing fasting glucose levels. The addition of GLP-1 RAs to basal insulin can often achieve glycemic control with the additional advantage of weight loss, a combination not seen with the addition of prandial insulin to basal insulin.
When used as add-on to basal insulin in appropriate patients, GLP-1 RA treatment can be particularly effective for postprandial glucose excursions, while having advantageous effects upon weight and satiety, and minimal risk of severe hypoglycemia. This may give the primary care provider strong rationale for consideration of this class of agents when adequate control is not achieved with other agents.
Louis Kuritzky, MD, is clinical assistant professor emeritus in the Department of Community Health and Family Medicine at the University of Florida in Gainesville, FL..
Timothy Reid, MD, is a board-certified family physician practicing at the Mercy Diabetes Center in Janesville, WI.
ACKNOWLEDGMENTS
The contents of the paper and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. The authors contributed to the writing of this manuscript, including critical review and editing of each draft, and approval of the submitted version. The authors received writing/editorial support in the preparation of this manuscript provided by Katherine Roberts, PhD, of Excerpta Medica, funded by Sanofi US, Inc. The authors of this manuscript have elected to not receive the honorarium offered by Consultant for publication of this article in the journal.
Disclosure
Louis Kuritzky, MD, has held/holds consultant or advisor roles for Boehringer Ingelheim, Janssen, Lilly, NovoNordisk, and Sanofi-Aventis. T
Timothy Reid, MD, has held/holds speaker and consultant roles for BMS/AstraZeneca, Novo Nordisk, Sanofi-Aventis, Janssen, Boehringer Ingelheim/Lilly, and Lilly.
(References on next page)
References:
1. Mudaliar S, Henry RR. The incretin hormones: from scientific discovery to practical therapeutics. Diabetologia. 2012;55(7):1865-1868.
2. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 Suppl 2:S279-S284.
3. Edwards KL, Stapleton M, Weis J, Irons BK. An update in incretin-based therapy: a focus on glucagon-like peptide-1 receptor agonists. Diabetes Technol Ther. 2012;14(10):951-967.
4. Fonseca VA, Varado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo- controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012;35(6):1225-1231.
5. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481.
6. Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252-258.
7. Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33(6):1255-1261.
8. Nauck M, Stewart M, Perkins C, et al. HARMONY 2 results at week 52 primary endpoint: once-weekly albiglutide monotherapy in drug naïve patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Paper presented at 49th Annual EASD Barcelona 2013. September 23-27, 2014; Barcelona, Spain. Abstract 55-LB.
9. Umpierrez G, Tofé Povedano S, Pérez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168-2176.
10. Raccah D. Efficacy and safety of lixisenatide in the treatment of type 2 diabetes mellitus: a review of phase III clinical data. Expert Rev Endocrinol Metab. 2013;8(2):105-121.
11. Bolli GB, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2014;31(2):176-184.
12. Ahrén B, Leguizamo Dimas A, Miossec P, et al. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013;36(9):2543-2550.
13. Rosenstock J, Raccah D, Korányi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36(10):2945-2951.
14. Rosenstock J, Hanefeld M, Shamanna P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications. 2014;28(3):386-392.
15. Yu Pan C, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M- Asia). Diabetes Metab Res Rev. 2014;30(8):726-735.
16. Pinget M, Goldenberg R, Niemoeller E, et al. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab. 2013;15(11):1000-1007.
17. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84-90.
18. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47.
19. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055.
20. Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431-439.
21. Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464-473.
22. Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301-1310.
23. Ahrén B, Johnson SJ, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141-2148.
24. Pratley R, Stewart M, Cirkel D, et al. HARMONY 4: 52-wk efficacy of albiglutide (Albi) vs. insulin glargine (Glar) in patients (pts) with T2DM. Paper presented at American Diabetes Association 73rd Scientific Sessions; June 21-25; Chicago, IL. Abstract 54-LB.
25. Home P, Stewart M, Mallory J, et al. Harmony 5 year 3 results: albiglutide vs. placebo and vs. pioglitazone in triple therapy (background metformin and glimepiride) in people with type 2 diabetes. Paper presented at EASD 2014 Virtual Meeting. Abstract 963-P.
26. Home P, Stewart M, Yang F, et al. 52-week efficacy of albiglutide vs placebo and vs pioglitazone in triple therapy (background metformin and glimepiride) in people with type 2 diabetes: HARMONY5 study. Paper presented at 49th Annual EASD Barcelona 2013. September 23-27, 2014; Barcelona, Spain. Abstract 58-LB.
27.
Reusch J, Stewart MW, Perkins CM, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled, trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab. 2014;16(12):1257-1264.
28. Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289-297.
29. Leiter LA, Carr MC, Stewart M, et al. 8: once weekly (QW) GLP1 agonist albiglutide (Albi) vs. sitagliptin (Sita) in type 2 diabetes (T2D) pts with renal impairment (RI): week 26 results. Diabetes. 2013;62(Suppl 1):A17.
30. Nauck MA, Weinstock RS, Umpierrez GE, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149-2158.
31. Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349-1357.
32. Giorgino F, Benroubi M, Sun JH, et al. Efficacy and safety of once-weekly dulaglutide vs. insulin glargine in combination with metformin and glimepiride in type
2 diabetes patients (AWARD-2). Diabetes. 2014;63(Suppl 1):A87.
33. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159-2167.
34. Jendle J, Rosenstock J, Blonde L, et al. Better glycemic control and less weight gain with once-weekly dulaglutide vs. once-daily insulin glargine, both combined with premeal insulin lispro, in type 2 diabetes patients (AWARD-4). Diabetes. 2014;63(Suppl 1):A246-A247.
35. DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092-1100.
36. Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762-768.
37. Derosa G, Maffioli P, Salvadeo SA, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12(3):233-240.
38. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083-1091.
39. Mathieu C, Ostenson CG, Matthaei S, et al. Using exenatide twice daily or insulin in clinical practice: results from CHOICE. Diabetes Ther. 2013;4(2):285-308.
40. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268-278.
41. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628-2635.
42. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32(7):1224-1230.
43. Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36(9):2489-2496.
44. Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes
inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013;36(9):2497-2503.
45. Seino Y, Min KW, Niemoeller E, et al. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917.
46. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317-2325.
47. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149.
48. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19(2):327-336.
49. Ross SA, Ekoé JM. Incretin agents in type 2 diabetes. Can Fam Physician. 2010;56(7):639-648.
50. Baynes KC. The evolving world of GLP-1 agonist therapies for type 2 diabetes. Ther Adv Endocrinol Metab. 2010;1(2):61-67.
51. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86(8):3717-3723.
52. Spellman CW. Pharmacology of GLP-1 agonists: describing the therapeutic potential to patients. J Am Osteopath Assoc. 2011;111(2 Suppl 1):eS10-eS14.
53. Nisal K, Kela R, Khunti K, Davies MJ. Comparison of efficacy between incretin-based therapies for type 2 diabetes mellitus. BMC Med. 2012;10:152.
54. Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68(5):557-567.
55. Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm. 2013;35(2):159-172.
56. Jellinger PS. Focus on incretin-based therapies: targeting the core defects of type 2 diabetes. Postgrad Med. 2011;123(1):53-65.
57. Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396-1405.
58. Lorenz M, Pfeiffer C, Steinsträsser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul Pept. 2013;185:1-8.
59. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103-112.
60. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care.2014;37 Suppl 1:S14-S80.
61. Berlie H, Hurren KM, Pinelli NR. Glucagon-like peptide-1 receptor agonists as add-on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2012;5:165-174.
62. Raccah D, Lin J, Wang E, et al. Once-daily prandial lixisenatide versus once-daily rapid-acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications. 2014;28(1):40-44.
63. Aquilante CL. Sulfonylurea pharmacogenomics in type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010;8(3):359-372.
64. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347(17):1342-1349.
65. Gallwitz B. Glucagon-like peptide-1 analogues for type 2 diabetes mellitus: current and emerging agents. Drugs. 2011;71(13):1675-1688.
66. Triplitt CL. Managing diabetes in patients with diabetes of long duration. Diabetes Educ. 2012;38(4 Suppl):23S-30S.
67. Lind M, Jendle J, Torffvit O, Lager I. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes. 2012;6(1):41-46.
68. Niswender K, Pi-Sunyer X, Buse J, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15(1):42-54.
69. Goldenberg R. Insulin plus incretin agent combination therapy in type 2 diabetes: asystematic review. Curr Med Res Opin. 2014;30(3):431-445.
70. Action to control cardiovascular risk in Diabetes (ACCORD) study. National Heart, Lung, and Blood Institute Web site. www.nhlbi.nih.gov/health-pro/resources/heart/accord-trial/questions-answers#trial. Published March 15, 2010. Accessed March 2015.
71. Vilsbøll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771.
72. Mannucci E, Dicembrini I. Incretin-based therapies and cardiovascular risk. Curr Med Res Opin. 2012;28(5):715-721.
73. Petrie JR. The cardiovascular safety of incretin-based therapies: a review of the evidence. Cardiovasc Diabetol. 2013;12:130.
74. Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (lixisenatide) (ELIXA). ClinicalTrials.gov Web site. http://clinicaltrials.gov/show/NCT01147250 . Accessed October29, 2014.
75. Exenatide study of cardiovascular event lowering trial (EXSCEL): a trial to evaluate cardiovascular outcomes after treatment with exenatide once weekly in patients with type 2 diabetes mellitus. ClinicalTrials.gov Web site. http://clinicaltrials.gov/show/NCT01144338 . Accessed October 29, 2014.
76. Ceriello A. Point: postprandial glucose levels are a clinically important treatment target. Diabetes Care. 2010;33(8):1905-1907.
77. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-742.
78. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
79. Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab. 2012;14(8):675-688.
80. Bolli GB, Owens DR. Lixisenatide, a novel GLP-1 receptor agonist: efficacy, safety and clinical implications for type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16(7):588-601.
81. American Diabetes Association. ADA/EASD/IDF Statement Concerning the Use of Incretin Therapy and Pancreatic Disease. June 28, 2013. www.diabetes.org/newsroom/press-releases/2013/recommendations-for.html . Accessed October 29, 2014.
82. U.S. Department of Health & Human Services. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. March 14, 2013. www.fda.gov/Drugs/DrugSafety/ucm343187.htm Accessed October 29, 2014.
83. European Medicines Agency. Investigation into GLP-1 based diabetes therapies concluded: no new concerns for GLP-1 therapies identified on the basis of available evidence. July 26, 2013. www.ema.europa.eu/docs/ en_GB/document_library/Press_release/2013/07/WC500146619.pdf. Accessed October 29, 2014.
84. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med. 2014;370(9):794-797.


