Anticholinergic Syndrome: Presentations, Etiological Agents, Differential Diagnosis, and Treatment
Introduction
The prescription of medications with anticholinergic properties to older patients must be undertaken judicially since the elderly have decreased cholinergic reserves and are prone to dementias (vascular, multi-infarct, and Alzheimer’s) and other conditions that are often worsened by anticholinergic medication1-4 (Table I). The decreased cholinergic reserve in older persons results in them being more susceptible to the side effects of anticholinergic medications, which include cognitive decline, impaired homeostatic regulation, and delirium. They are also at a higher risk for developing an anticholinergic toxicity  syndrome.3-6 Due to the large number of medications used by the elderly (average, 5-10 prescriptions), it is often the additive effects of these medications that lead to acute anticholinergic toxicity.3,6-11 Currently, there are over 600 medications identified as having some degree of serum anticholinergic activity (SAA).3,7,12 In addition, patients can be exposed to the anticholinergic effects of street drugs, over-the-counter medications, and herbal products/medication.3,12-15
syndrome.3-6 Due to the large number of medications used by the elderly (average, 5-10 prescriptions), it is often the additive effects of these medications that lead to acute anticholinergic toxicity.3,6-11 Currently, there are over 600 medications identified as having some degree of serum anticholinergic activity (SAA).3,7,12 In addition, patients can be exposed to the anticholinergic effects of street drugs, over-the-counter medications, and herbal products/medication.3,12-15
Identifying a patient with anticholinergic toxicity can be difficult since its presentation is often similar to the delirium caused by other conditions (eg, infections, benzodiazepine withdrawal, metabolic disturbance). It may also appear similar to other medication-induced symptoms such as neuroleptic malignant syndrome (NMS) and central serotonin syndrome.16-19 The degree of anticholinergic toxicity can range from producing minor symptoms, which may be mistaken as the “normal changes of aging” (eg, minor cognitive impairment), to the common benign side effects of medications (eg, constipation, dry mouth) to severe symptoms (eg, acute agitated delirium with hallucinations, hyperthermia, coma, death). The correct diagnosis of anticholinergic toxicity depends on the treating physicians’ awareness of the condition, recognition of its symptoms, appreciation of the various autonomic processes affected by the neurotransmitter/hormone acetylcholine, and an understanding of the potential additive anticholinergic effects of various medications.
Why Older Persons Are Susceptible to Anticholinergic Toxicity
Many medications with anticholinergic properties are used to treat diseases in older persons (eg, urinary incontinence, emphysema). More than 80% of the elderly population report at least one chronic disease, with the average community-dwelling elderly individual reporting three to five chronic medical conditions.6 Nursing home residents report significantly more chronic illnesses and take more medications than the community-dwelling elderly.6,10 It has been estimated that 51% of the general population use some medication with anticholinergic properties on a regular, if not a daily, basis.20 Studies focused on the elderly have found a prevalence of 10-40% of community-dwelling elderly and 30-60% of nursing home residents who are taking at least one medication with significant anticholinergic properties, and that approximately 7% of community-dwelling elderly and 10-17% of nursing home residents routinely use multiple anticholinergic medications.4-6,8,20 In a study by Remillard8 using the health insurance databases of the province of Saskatchewan, Canada, it was found that 25% of the elderly persons prescribed anticholinergic medication were receiving doses in the “high to excessive dose range.”
Many of the medications used to treat chronic conditions cannot be readily stopped and/or are needed to prevent potential life-threatening conditions. Examples of such classes of medications that frequently cause strong anticholinergic effects include antiemetics, antispasmodics, bronchodilators, antiarrhythmics, antihistamines, various analgesics, antihypertensives, antiparkinsonian agents, corticosteroids, skeletal and smooth muscle relaxants, antiulcer drugs, and psychotropics1,20,21 (Table II). Many of these medications, such as anticholinergic eye drops, furosemide, digoxin, cimetidine, and prednisolone, are not commonly thought to have systemic anticholinergic effects.6,21-23 This results in these medications being prescribed without the realization that an additive anticholinergic load is occurring.
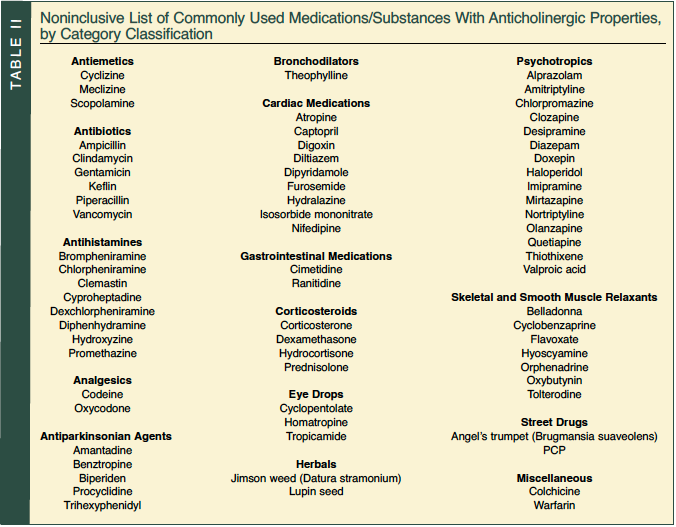
Physicians may not realize that eye drops can be a significant factor in anticholinergic toxicity. Eye drops are usually very concentrated; are directly absorbed, bypassing first-pass liver metabolism; and may be more readily absorbed by the elderly due to the increased surface area/permeability of capillary networks around the eye (eg, “bags under the eyes”). Older patients often have more difficulty in the delivery of a standard measured dose of an eye drop because of tremor, which results in more medication being used than was intended.6,23,24
In addition to the increased number and the type of medications they are prescribed, the elderly are also susceptible to developing elevated drug levels due to changes in metabolism (eg, individual pharmacokinetic and pharmacodynamic variability), increased blood-brain barrier permeability, and decreased drug clearance related to aging.5-7,10,16-20,25,26 As people age, the efficiency of proteins that are required for the production of acetylcholine, as well as the number of acetylcholine receptors, decreases.5,9,26 All of these factors lead to elderly persons experiencing twice as many adverse drug reactions as the general population overall and, in particular, two to three times more anticholinergic reactions than the general population.7,8
Alzheimer’s disease is the most common form of dementia in older persons. Individuals with dementia are at increased risk for being sensitive to anticholinergic medicines due to the age-associated decline in acetylcholine, as well as the loss of cholinergic cell bodies (eg, loss of cholinergic cell bodies in the nucleus basalis of Meynert, which is an associated finding in Alzheimer’s disease).2,7,26 Although there are many proposed mechanisms for how Alzheimer’s disease progresses and causes cognitive decline, there is a positive correlation between decreased levels of acetylcholine and diminished cognitive function.2,3,27 This also explains why medications with strong anticholinergic effects significantly worsen individuals with Alzheimer’s dementia, who already have diminished acetylcholine levels.
Acetylcholine and Its Mechanisms of Transmission
Acetylcholine was classically viewed as a neurotransmitter found in peripheral neurons (eg, parasympathetic pathways, neuromuscular junction), as well as in the central nervous system (eg, forebrain, midbrain, brainstem).5,28,29 Recently, more recognition of and research into the hormonal (nonneuron–released) effects of acetylcholine is taking place. The 2005 Goodman and Gilman’s The Pharmacological Basics of Therapeutics has added an entire chapter dedicated to the nonneuronal cholinergic effects of acetylcholine.30 Several recent international conferences have been dedicated to discussion of nonneuron acetylcholine usage by the body.29,30 By today’s standards, acetylcholine would be classified as a “neurohumoral transmitter” since it can function as a neurotransmitter, local cell signaling agent, or a hormone.29-31
In terms of neurological signaling, there are predominately two distinct receptor types for acetylcholine: muscarinic and nicotinic.5,6,31,32 Muscarinic receptors are primarily found on the autonomic effector cells that are innervated by postganglionic parasympathetic nerves throughout the brain.31 Nicotinic receptors are primarily located in the autonomic ganglion and at the neuromuscular junction and are not affected by atropine-like medicines except when they reach relatively high concentrations, at which point they cause a partial receptor blockade.6,31 The muscarinic receptors are primarily responsible for the therapeutic and side-effect profile seen with most traditional anticholinergic medications.5,6,31-33 Many medications with anticholinergic properties, such as atropine, are nonselective in their blockade of the five subtypes of muscarinic receptors; however, more selective agents are being developed and are starting to be used (eg, darifenacin and imidafenacin for  treatment of overactive bladder, which has greater selectivity for the muscarinic 3 receptor subtype; imidafenacin is currently in phase 3 trials).6,30-34
treatment of overactive bladder, which has greater selectivity for the muscarinic 3 receptor subtype; imidafenacin is currently in phase 3 trials).6,30-34
In the central nervous system, major cortical cholinergic tracts project from the nucleus basalis of Meynert and the substantia innominata of the basal forebrain.28,32 These projections are important for memory function and attention.6,20,28,32,33 Blockade of these projections causes the cognitive changes that occur with anticholinergic medications. Peripheral muscarinic receptors are located in the heart, lungs, gastrointestinal (GI) tract, eyes, secretory glands, and skin. They explain the multitude of peripheral side effects seen with anticholinergic medications.6,29-31
Central Anticholinergic Toxicity
Central anticholinergic syndrome often goes unrecognized since the symptoms of the condition often do not present in a well-defined pattern and may present with a wide array and severity of symptoms (eg, anticholinergic psychosis)12 (Table III). The general hallmark of the condition is some degree of delirium concurrently presenting with diminished parasympathetic function12,22,35 (Table IV). To make the diagnosis, physicians need to observe both central and peripheral nervous system symptoms.12,22,35 To further confound the diagnosis, temporal fluctuations of symptoms with a pattern of waxing and waning of individual symptoms can occur.12,35
 Symptoms produced from blockade of peripheral acetylcholine receptors include hyposalivation/decreased secretions, slowed gastric motility, urinary retention (especially in men with an enlarged prostate), mydriasis resulting in blurred vision or the acute precipitation of narrow-angle glaucoma, heat intolerance usually resulting in hyperthermia, and cardiovascular changes such as tachycardia and widened pulse pressures.3,4,6-8,12,35-37 Thus, the classic patient presents with dry skin, flushed face, dry mouth, constipation, urinary retention, abdominal distress, tachycardia, widened pulse pressure, and dilated pupils that are poorly reactive to light.12,35-37 In terms of skeletal muscle changes, patients have poor coordination, ataxia, dysarthria, and the potential for increased muscular tone followed by profound muscular weakness/flaccid paralysis and myotonic twitching.12,35 Severe cases of toxicity can result in cardiac arrhythmias/circulatory collapse and gastric ileus.6,22
Symptoms produced from blockade of peripheral acetylcholine receptors include hyposalivation/decreased secretions, slowed gastric motility, urinary retention (especially in men with an enlarged prostate), mydriasis resulting in blurred vision or the acute precipitation of narrow-angle glaucoma, heat intolerance usually resulting in hyperthermia, and cardiovascular changes such as tachycardia and widened pulse pressures.3,4,6-8,12,35-37 Thus, the classic patient presents with dry skin, flushed face, dry mouth, constipation, urinary retention, abdominal distress, tachycardia, widened pulse pressure, and dilated pupils that are poorly reactive to light.12,35-37 In terms of skeletal muscle changes, patients have poor coordination, ataxia, dysarthria, and the potential for increased muscular tone followed by profound muscular weakness/flaccid paralysis and myotonic twitching.12,35 Severe cases of toxicity can result in cardiac arrhythmias/circulatory collapse and gastric ileus.6,22
Central cholinergic effects can range from sedation, cognitive slowing, and confusion to more severe effects such as agitation, hallucinations (visual and auditory), and coma.6,12,20,27,35-37 Somnolence and coma occur in less than one-third of severe cases of anticholinergic toxicity. When coma does occur, it is generally late in the course of the syndrome.12
Research studies have found that administering an anticholinergic agent such as scopolamine to either a healthy young adult or to someone over the age of 65 results in reduced hippocampal activation on functional magnetic resonance imaging, impaired attention, diminished memory performance (relative sparring of implicit memory), and psychomotor slowing.5,20,33 Reports of anticholinergic psychosis without accompanying peripheral signs have been reported to occur following intoxication with anticholinergic eye drops.23
Higher levels of SAA are associated with delirium and impaired cognitive performance, especially in individuals with mild-to-moderate pre-exposure dementia.1,9,26,27,33,38-41 Mulsant et al,33 in a study looking at the cognitive effects of anticholinergic drugs, found that a significant association exists between SAA and the Mini-Mental State Examination (MMSE) score. Individuals who had SAA of 2.80 pmol/mL or higher were 13 times (P < 0.05; confidence interval, 1.08-152.39) more likely to have MMSE scores of 24 or lower.33 Flacker et al27 showed that the association between delirium and SAA had an odds ratio of 1.95 (P = 0.003). For every quintile increase in SAA, there was a 2.38-times increase in the likelihood of a delirium developing (7.7% for the lowest quintile to 61.5% for the highest).27 Not surprisingly, the severity of the delirium increased proportionately as the SAA increased. Considering that 10-38% of elderly medical inpatients experience delirium, and that delirium has an associated mortality rate of 5-60%, it is clear that the anticholinergic side effects/toxicity of medications can significantly increase mortality in older patients.27,39,42-44
Determining Toxicity
Although there are serum assays for measuring anticholinergic levels (eg, the Tune and Coyle assay method), they do not appear to accurately predict those who have anticholinergic toxicity from those who do not.7 This is because individual patients have varying physiological predisposition (eg, level of cholinergic reserve, permeability of the blood-brain barrier) to developing anticholinergic toxicity.6,7,9,20,38,41 Individuals who have taken anticholinergic agents such as tricyclic antidepressants for a protracted period of time may develop tolerance and not experience symptoms even at high blood levels.7 Also, individuals may have varying levels of cholinergic reserve (eg, enzyme levels, receptor/neuron number and receptor activity), so it is possible for someone to become toxic at a level lower than expected.6,7,9,26 In addition, individuals who were stable on a particular medication regimen may suddenly become toxic secondary to a new illness, which may cause the production of endogenous anticholinergic compounds, disrupt the signaling pathways of cholinergic neurons, or result in the introduction of a new medication that produces additional anticholinergic effects.27-29,38,39
Unlike other medication-induced states such as NMS and central serotonin syndrome, there is no algorithmic system to determine the severity of the toxicity the patient is experiencing. Many physicians still depend on the mnemonic that they learned as residents to determine if a patient is displaying muscarinic autonomic anticholinergic symptoms (ie, “red as a beet, dry as a bone, blind as a bat, hot as a hare, mad as a hatter”).6 Relying solely on this mnemonic can result in symptoms such as tachycardia, widening pulse pressure, and ataxia being overlooked or not appreciated as potential harbingers of the syndrome.12,35
Differentiation of Drug Toxicities
Many drug toxicity syndromes such as NMS and central serotonin syndrome have an overlapping symptom profile, which can make correct diagnosis difficult. In addition, many of the medications that cause these syndromes have multiple pharmaceutical properties. For example, quetiapine has anticholinergic effects, is a neuroleptic, and has been associated with serotonin syndrome when taken in combination with other medications.16,17,21,45,46
Central serotonin syndrome can present with a myriad of potential symptoms and has three different screening algorithms: Sternbach, Radomski, and Dunkley.18 In general, symptoms of serotonin toxicity consist of changes in mental status, neuromuscular abnormalities, and autonomic dysfunction. Specific symptoms of central serotonin syndrome include confusion, hypomania, agitation, diaphoresis, skin flushing, shivering, low-grade fever, clonus, rigidity, hyperreflexia, hyperactive GI motility, and possible mydriasis.18,19,47 Both anticholinergic syndrome and central serotonin syndrome present with altered mental status, tachycardia, mydriasis, and fever.18,47 Anticholinergic toxicity can be distinguished from central serotonin toxicity by presenting with lack of sweating, diminished salivation, diminished GI motility, and widened pulse pressure.18,47
NMS often presents with the triad of rigidity (eg, lead-pipe), high fever, and confusion. It can also have the associated laboratory finding of an elevated creatine phosphokinase caused by muscle breakdown related to the rigidity.16,17,47 As with serotonin syndrome, there are many different algorithmic diagnostic criteria (eg, DSM-IV-TR, Pope, Adityanjee).17 Additional symptoms seen with NMS include diaphoresis, dysphagia, tachycardia, diminished reflexes, blood pressure fluctuations, leukocytosis, and incontinence. Anticholinergic toxicity and NMS can share the symptoms of fever, confusion, tachycardia, and potentially decreased bowel sounds. They are distinguished from each other by anticholinergic toxicity presenting with mydriasis and decreased secretions.16,17,47
Treatment
Treatment for anticholinergic toxicity primarily involves reducing or stopping anticholinergic agents, engaging in supportive therapy (eg, IV hydration, nutrition, and, if needed, medications for symptoms of delirium), admission to a monitored bed due to cardiac complications, and in severe cases the use of physostigmine to reverse the effects of the anticholinergic agent.12,35-37 Physostigmine is a tertiary amine that rapidly crosses the blood-brain barrier and is an acetylcholinesterase inhibitor. Physostigmine improves both the central and peripheral symptoms associated with anticholinergic toxicity.12,35 It has been used to treat anticholinergic delirium since the mid-1800s and is preferred over other cholinergic agents due to its rapid onset of action and short half-life.12,14,33,48 Because physostigmine has a short half-life, a patient may need to be given repeated administrations of the medication if symptoms recur.12,14,35,48 If symptoms are due to anticholinergic toxicity, improvement occurs rapidly, often within minutes.12,35 The usual dose of physostigmine is 1-2 mg given IM or 0.02 mg/kg IV, with the patient being observed for 30 minutes afterwards for either improvement or the development of cholinergic symptoms such as arrhythmias.12,14,35 Rapid improvement following physostigmine injection is evidenced by improved cognition, decreased tachycardia, and dryness of the mouth.12 Mydriasis may take days to fully resolve, even with continued physostigmine treatment.12 Pilocarpine, a miotic medication, is helpful to determine if mydriasis is related to anticholinergic effects or another cause.14
 Just as excessive anticholinergic activity can lead to negative health effects, too much cholinergic activity can also result in potentially life-threatening complications.12,48 The pharmacology of physostigmine is very complex due to the multitude of effects that acetylcholine produces on preganglionic, postganglionic, somatic motor, and central nervous system receptors.12 Physostigmine has a predominantly parasympathetic effect, but by virtue of its preganglionic stimulation, it can exert sympathetic influences as well12 (Table V). Signs of physostigmine/cholinergic toxicity are, in general, the reverse of those seen with anticholinergic toxicity and consist of bradycardia, hypotension, hypothermia, increased secretions (eg, lacrimation, salivation, rhinorrhea, bronchial secretions), muscle weakness, dizziness, diaphoresis, miosis, nausea, and potential seizures.12,15,48 Parasympathetic activation of the cardiovascular system (eg, heart block) requires careful monitoring. Precipitation of an acute refractory asthmatic episode following physostigmine administration may also occur if the dosing is too high or too rapidly administered.12,31,35 If too much physostigmine is given or if cholinergic complications arise (eg, heart block, asthma, seizures), the symptoms can be reversed by giving 0.5 mg of atropine for each milligram of physostigmine administered, as well as providing other appropriate treatments for the specific complication.12,35
Just as excessive anticholinergic activity can lead to negative health effects, too much cholinergic activity can also result in potentially life-threatening complications.12,48 The pharmacology of physostigmine is very complex due to the multitude of effects that acetylcholine produces on preganglionic, postganglionic, somatic motor, and central nervous system receptors.12 Physostigmine has a predominantly parasympathetic effect, but by virtue of its preganglionic stimulation, it can exert sympathetic influences as well12 (Table V). Signs of physostigmine/cholinergic toxicity are, in general, the reverse of those seen with anticholinergic toxicity and consist of bradycardia, hypotension, hypothermia, increased secretions (eg, lacrimation, salivation, rhinorrhea, bronchial secretions), muscle weakness, dizziness, diaphoresis, miosis, nausea, and potential seizures.12,15,48 Parasympathetic activation of the cardiovascular system (eg, heart block) requires careful monitoring. Precipitation of an acute refractory asthmatic episode following physostigmine administration may also occur if the dosing is too high or too rapidly administered.12,31,35 If too much physostigmine is given or if cholinergic complications arise (eg, heart block, asthma, seizures), the symptoms can be reversed by giving 0.5 mg of atropine for each milligram of physostigmine administered, as well as providing other appropriate treatments for the specific complication.12,35
Often, patients with delirium are treated with neuroleptic medication in hopes of obtaining both symptomatic and functional improvement. Due to recent black box warnings concerning neuroleptics causing an increasing risk of mortality in individuals with dementia, many physicians have become hesitant to prescribe these medications.49,50 When these medications are used to treat patients with anticholinergic delirium, the risk of the delirium should outweigh the risk of the treatment (eg, delirium causing unmanageable psychosis, harm to others, self-harm behavior, or increased morbidity due to prolonged hospitalization, poor nutrition, or increased fall risk).49,50 In such cases, the neuroleptic medication used needs to be chosen carefully.39,49-51 Many of the typical and atypical neuroleptics, such as thioridazine, quetiapine, and olanzapine, bind to muscarinic receptors and may worsen or slow the recovery of an anticholinergic-induced delirium.3,46,52 If a neuroleptic medication is to be used in these cases, it may be best to start with low-dose risperidone or haloperidol since these medications have been found to have lower to no anticholinergic properties as compared to other neuroleptics, and have various ways to be administered (eg, haloperidol comes in PO, IM, or IV preparations), an often critical factor in the treatment of patients with agitation and delirium.3,44,51,52
Summary
Considering the more than 600 medications that have anticholinergic properties, the physiological variables that exist in older patients, and the exacerbation that can occur from various diseases and medications, the diagnosis of anticholinergic toxicity can often be overlooked, particularly if its onset is subtle and gradual. The severity of anticholingeric side effects, if not those of blatant toxicity, is extremely variable. Minor forms can result in a presentation of dry mouth and minor cognitive confusion, while severe forms may present with fever, confusion, coma, and death. When anticholinergic toxicity states are recognized, they can be effectively treated by stopping the offending agents, administering and titrating physostigmine, treating delirium with medications such as risperidone or haloperidol, and providing supportive care and monitoring.
The authors report no relevant financial relationships.
Dr. Ryan Hall is an Affiliate Instructor, University of South Florida, Tampa, Assistant Professor of Psychiatry, University of Central Florida College of Medicine, Orlando, and is a 2006 Rappeport Fellow; Dr. Richard Hall is Professor of Psychiatry, University of Central Florida College of Medicine, Courtesy Clinical Professor of Psychiatry, University of Florida, Gainesville, and Affiliate Professor of Psychiatry, Department of Psychiatry and Behavioral Medicine, University of South Florida; and Ms. Chapman is Research Assistant to Dr. Richard Hall and Dr. Ryan Hall.


