Wound Infection: Primary Considerations
Wound Care Clinic
How to Treat, When to Refer
This article is adapted from Wound Bed Preparation: It’s About Time, an HMP Communications, LLC publication provided to readers of Ostomy Wound Management, June 2005.
Wound infection delays wound closure. It prolongs the inflammatory phase of healing and often causes distress and discomfort for patients. Increased bacterial burden may not be obvious in all wounds; in the chronic wound, signs of infection may be quite subtle and, therefore, overlooked. Prompt identification and management of infection is essential in the management of chronic wounds.
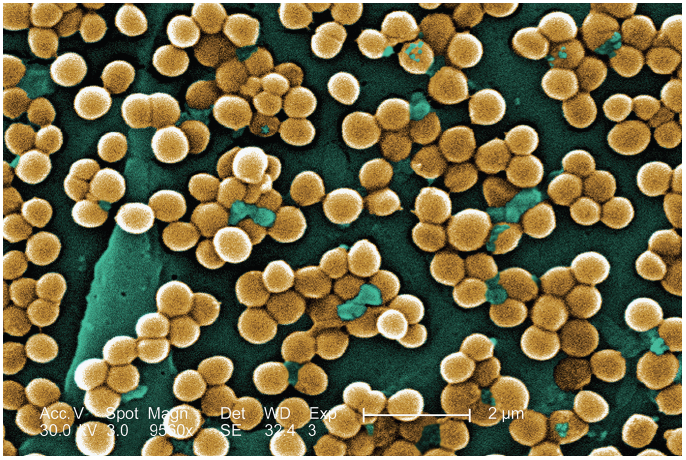
FIGURING OUT WHAT YOU ARE DEALING WITH
All chronic wounds contain bacteria, and their presence in the wound does not necessarily indicate that infection has occurred or has impaired wound healing.1,2 Bacterial colonization alone is not clinically significant and should not be confused with a clinical diagnosis of wound infection. However, when bacteria are sufficiently virulent to compromise host response, wound healing is affected.
Bacterial levels. Bacterial involvement within a wound can be divided into four categories:
•Contamination refers to the presence of non-multiplying bacteria within a wound and accounts for the majority of the micro-organisms present on the wound surface.3
•Colonization refers to the presence of bacteria that are multiplying but are producing no host reaction.
•Critical colonization may be defined as an increased bacterial burden (multiplication of organisms) within the wound that initiates the body’s immune response locally but not systemically and results in delayed healing.
•Infection is the presence of multiplying bacteria that cause an associated host reaction.
A pathogenic micro-organism may initially colonize a wound without inducing a host reaction. However, as the bacterial burden increases, the colonized wound gradually is transformed into an infected wound.4 Pathogenic micro-organisms responsible for wound infection prolong wound healing by destroying cells by competing for available oxygen supplies within the wound; releasing toxins that damage tissue locally, causing necrosis and pus formation; and releasing toxins into the blood stream that may cause toxemia.
Experimental evidence indicates that, regardless of the type of organism, substantial impairment of wound healing occurs when the wound bed contains between 105 and 106 organisms per gram.2 However, the number of organisms in a wound may not necessarily be as critical as the type and pathogenicity of the organisms in the wound. Furthermore, increased bacterial load often increases the amount of wound exudate, requiring effective management to avoid providing a venue for increased bacterial infection.
Is it infected? Most clinicians are aware of the “classic” signs and symptoms of infection (advancing erythema, fever, warmth, edema, pain, purulence) that often are seen in the acute wound or the severe chronic wound. However, many clinicians often overlook the subtle “secondary” signs and symptoms associated with infection in the chronic wound. Secondary signs of infection include:
•Delayed healing
•Change in color of wound bed
•Friable granulation tissue
•Absent or abnormal granulation tissue
•Increased or abnormal odor
•Increased serous drainage
•Increased pain at wound site.5,6
What is it? How best to determine the type(s) of offending organism(s) is up for debate. The established practice of diagnosing wound infection by isolating bacteria in the wound bed from a single bacteriological swab may be considered inadequate and misleading.7 Microbiologists recommend performing needle aspiration or biopsies utilizing several areas in the wound bed, but this invasive procedure is rarely performed in clinical practice. The presence or absence of the objective clinical signs rather than bacteriological analysis in isolation remains the best way to establish the presence of infection—that, and the consideration of a number of additional variables such as the amount of necrotic or slough tissue present in the wound bed, the number of organisms present, bacterial pathogenicity, and host factors.3
The host with the most resistance. Host monitoring is a critical aspect of wound assessment and management. Local factors that increase the likelihood of wound infection include wound size, depth, and duration. A larger wound is associated with greater host impairment during wound healing and, consequently, a greater risk of infection. Vascular status is equally as important—wounds with a reduced arterial pressure will remain unhealed.8 The extent of wound perfusion also must be considered, because an inadequately perfused wound is unlikely to show the typical signs of inflammation. Additional systemic factors include necrotic tissue, foreign bodies, metabolic disorders (eg, diabetes), and corticosteroids.
Biofilms.Sheets of soft adherent material (biofilms) are highly organized bacterial communities that allow individual organisms to interact with each other, exchanging nutrients and metabolites. Biofilms represent a foci of infection and bacterial resistance within the wound that protect the bacteria from the effects of antimicrobial agents, especially antibiotics and antiseptics.9 Recognition and management of biofilms remain controversial.
OVERCOMING OBSTACLES TO HEALING
Identifying and reducing the bacterial burden in the wound is an important component of preparing the wound for closure. The early detection and treatment of wound infection limits the amount of local tissue damage and minimizes disruption of the healing process.
The topical approach. An important aspect of preventing and/or healing wound infection is ensuring an appropriate moisture balance within the wound. This can be achieved using a moisture-balancing dressing that handles wound exudate and helps suppress bacterial growth.
Since the introduction of topical antiseptics 150 years ago, a wide range of antiseptics has been used to prevent and treat wound infections. Despite conflicting research, an important goal has emerged: to balance the effects of bacteria against the potential cytotoxicity of antiseptics. Plus, as bacteria become more resistant to antibiotics, interest grows in the selective use of topical antimicrobial agents.
If used correctly, topical antiseptic agents, despite their cytotoxic properties, can be effective antibacterial agents.3 Some iodine and silver preparations have bacteriocidal effects, even against multiresistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA).10,11 Furthermore, in contrast to antibiotics, which have a more specific mode of action and are effective against a narrower range of bacteria, broad-spectrum antimicrobial agents provide action across three target areas—the cell membrane, cytoplasmic organelles, and the bacteria’s nucleic acid.11
Antibiotics. The use of antibiotics should be restricted to situations for which they are absolutely necessary. Systemic antimicrobial therapy should be used when active infection cannot be managed with local therapy (eg, fever, underlying deep structure infection, and spreading cellulitis).3 Systemic infection, sepsis, or total host invasion has a high morbidity risk; multidrug therapy, including steroids, non-steroidal anti-inflammatory drugs, and interferon in addition to antibiotics and nutrition support, may be necessary.
Topical antibiotics may increase the potential for bacterial resistance and should be used prudently. Allergic reactions to topical antibiotics such as neomycin, framycetin, gentamicin, and sodium fusidate are common.12
CONCLUSION
All wounds contain bacteria but may not be infected; the infected wound stalls healing in the inflammatory stage. Although culturing the wound can determine the type of infectious organism and subsequently guide treatment, the clinician’s best tools are his or her ability to visual appraise for signs of infection before more invasive actions and stronger medications are initiated.
REFERENCES:
1. Kerstein MD. The scientific basis of healing. Adv Wound Care. 1997;10:30-36.
2. Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage. 1999;45:23-40.
3. Schultz GS, Sibbald GR, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Rep Regen. 2003;11(2):1-28.
4. Thompson P, Smith D. What is infection? Am J Surg. 1994;167:7S.
5. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care. 1994;3(4):
198-201.
6. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized wound infection. Wound Rep Regen. 2001;9(3):178-186.
7. Gilchrist B. Taking a wound swab. Nursing Times. 2000;96(4):2.
8. Carter SA. Role of pressure measurements. In: Berstein EF, ed. Vascular Diagnosis, 4th ed.
St. Louis, Mo.; Mosby;1993;486-512.
9. Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiology
Molecular Biology Review. 2000;64:847-867.
10. Lawrence JC. The use of iodine as an antiseptic agent. J Wound Care; 1998;7(8):421-425.
11. Sibbald, RG, Browne AC, Coutts P, Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage. 2001;47:38-43.
12. Bajaja AK, Gupta SC. Contact hypersensitivity to topical antibacterial agents. Int J Dermatol. 1986;25:103-105.
Editor’s note: This article is the third in a series on wound management. The first
(“Assessing and Managing a Moist Wound Environment,” CONSULTANT, March 2012, page 214) and second (“Wound Bed Preparation,” CONSULTANT, June 2012, page 459) articles are available on our web site at http://www.Consultant360.com.


