Peer Reviewed
Retinal Vein Occlusions: Diagnosis and Management
AUTHORS:
Leonid Skorin Jr, DO, OD, MS1 • Taylor Lauermann, BS2
AFFILIATIONS:
1Mayo Clinic Health System, Albert Lea, Minnesota
2Pacific University College of Optometry, Forest Grove, Oregon
CITATION:
Skorin L Jr, Lauermann T. Retinal vein occlusions: diagnosis and management. Consultant. 2020;60(10):3-5, 12, 16. doi:10.25270/con.2020.05.00016
Received January 8, 2020. Accepted April 10, 2020.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Leonid Skorin Jr, DO, OD, MS, Mayo Clinic Health System, 404 W Fountain St, Albert Lea, MN 56007-2437 (skorin.leonid@mayo.edu)
ABSTRACT: Retinal vein occlusion is divided into 3 types—central retinal vein occlusion (CRVO), hemiretinal vein occlusion (HRVO), and branch retinal vein occlusion (BRVO). Patients with these conditions usually present with a sudden painless loss of vision in one eye. While the incidence of these conditions is low, they can have devastating vision-related complications. This article reviews the risk factors and ocular signs of retinal vein occlusions. Proper management and treatment plans are described for the different types of retinal vein occlusions.
KEYWORDS: Retinal vein occlusion, central retinal vein occlusion, hemiretinal vein occlusion, branch retinal vein occlusion
Retinal vein occlusion is divided into 3 different types—central retinal vein occlusion (CRVO), hemiretinal vein occlusion (HRVO), and branch retinal vein occlusion (BRVO). Patients with these conditions usually present with a sudden painless loss of vision in one eye. One population-based study has reported the prevalence of CRVO as 0.1% and of BRVO as 0.6% of the population.1 While the incidence of these conditions is low, they can have devastating visual complications.1
HOW VEIN OCCLUSIONS AFFECT THE EYE
When a vein occlusion occurs, it causes stasis of blood drainage within the eye. The stasis often results from thrombus formation due to an artery compressing a vein. This blockage leads to hemorrhaging, damage of the retinal vessels, and ischemia. The origin of the occlusion and its effects depends on the area of occlusion. If the occlusion occurs in smaller veins of the retina, it is referred to as a BRVO, and vision loss may be less extensive. If the occlusion occurs in larger veins, such as the central retinal vein, vision loss is often devastating and widespread, because the whole retina is involved. With each type of vein occlusion, the cause of the vision loss is due to damage of the vasculature, which ultimately leads to ischemia. Ischemia causes areas of the retina to become nonfunctional due to a lack of nutrient supply. Ultimately, this leads to permanent loss of vision. Another cause of vision loss in vein occlusions is edema. Edema results from leakage of fluid from the involved vasculature. This fluid can cause separation of the retinal layers, which causes vision loss. If edema occurs within the macula, decrease in vision can be significant.1
It is important to consider systemic health when examining a patient with a vein occlusion. Some of the most common conditions associated with vein occlusions include hypertension, diabetes, increased body mass index, generalized systemic vascular disease, glaucoma, autoimmune and inflammatory conditions, and clotting disorders. If a patient with any of these conditions presents with a vein occlusion of the retina, it is important to consider that this patient is at a higher risk of having a future devastating systemic complication.2
ISCHEMIC VS NONISCHEMIC
When assessing retinal vein occlusions, specifically CRVO, it is also important to classify the occlusion as either ischemic or nonischemic. Nonischemic CRVO often presents with unilateral sudden painless loss of vision. The patient’s visual acuity level will be better than 20/200, and examination of the posterior pole of the fundus will reveal dot/blot hemorrhages in all 4 quadrants with mild dilation and tortuosity of the vessels. Resolution of nonischemic CRVO often begins within 6 to 12 months. The patient’s vision usually returns to near-normal levels as long as it does not progress into an ischemic CRVO. There is a 15% chance of going from a nonischemic to ischemic CRVO within the first 4 months, and a 34% chance of this occurring within 3 years.2
Ischemic CRVO also presents with unilateral sudden painless loss of vision. In these patients, acuity levels will be reduced to worse than 20/200. Posterior pole assessment will reveal retinal vessel tortuosity with dilated vessels. Dot/blot hemorrhages will be present along with flame-shaped hemorrhages in all 4 quadrants. The patient may also have areas of nonperfusion presenting as cotton wool spots (Figure 1). Patients with an ischemic CRVO have a poor prognosis due to the ischemia within the macula. These patients typically do not show improvement and need to be monitored regularly for neovascularization. Neovascularization is worrisome, because the new blood vessels can grow at the optic nerve, retina, and on the iris, causing neovascular glaucoma. Neovascular glaucoma is most likely to occur in the first 2 to 5 months (100-day glaucoma) after initial presentation but may occur any time over the 2-year follow up period.2

Figure 1. Fundus photo of the right eye with infratemporal BRVO showing dot/blot and flame hemorrhages with cotton wool spots (arrow).
Due to the stark difference in outcomes between nonischemic and ischemic CRVO, it is important to be able to differentiate between the two. Two tests that aid in differentiation are pupil testing and electroretinography (ERG). It is highly likely that the vein occlusion is ischemic if the patient presents with a relative afferent pupillary defect (Marcus Gunn pupil) and has reduced amplitude of the B wave on ERG. Using those two tests helps in differentiating 97% of cases.2,3 Determining the classification of a CRVO as ischemic significantly changes the path of treatment and management for the patient; therefore, this needs to be determined early in the course of the condition.
TYPES OF RETINAL VEIN OCCLUSIONS
CRVO is one of the primary causes of sudden unilateral painless vision loss in adults. The initial visual acuity after occurrence of the CRVO determines prognosis. If a patient presents with visual acuity of 20/60 or better, it will likely remain at this level. If the acuity level is 20/80 to 20/200, the acuity could remain stable, improve, or worsen. If the acuity level is worse than 20/200, improvement is unlikely. It is also important to determine whether ischemia is present, because ischemic CRVO has a more guarded prognosis and may develop neovascularization. In eyes with ischemia, there is a 50% chance of developing neovascularization of the iris, which often leads to neovascular glaucoma.4
A CRVO occurs due to the compression of the central retinal artery on the central retinal vein. Both the artery and vein share a common sheath of adventitia. This predisposes the vein to compression from the artery when certain factors are present such as diabetes and hypertension. This leads to thrombus formation and causes blockage of blood outflow, leading to stasis. This in turn causes damage to vessels within the retina and can lead to hemorrhaging.4
BRVO has a better prognosis then CRVO. Approximately 50% to 60% of eyes that have a BRVO will return to an acuity level of 20/40 or better. Similar to CRVO, the BRVO prognosis is determined by acuity level. There are no black-and-white prognostic acuity levels, but in general, if macular involvement is present, the prognosis is worse. Prognosis is also worse if there is at least 5 disc diameters of retinal ischemia. In those cases, there is a 36% chance of developing neovascularization of the disc or retina. Also, in 2% of eyes with a BRVO, neovascularization of the iris will occur.5
BRVO often results from multiple underlying mechanisms. One of the more common etiologies is arteriosclerosis of the retinal arteries due to hypertension. This can cause compression of veins from arteries at crossing points in the retina due to the common adventitial sheath that they share. A thrombus can occur at that crossing point, causing a BRVO. Another mechanism is hypercoagulability disorders, which can also lead to formation of a thrombus. When either of these mechanisms occurs, stasis of blood flow occurs, leading to damage of the involved retinal vessels and hemorrhaging.5
HRVO has a worse prognosis than BRVO but a better prognosis than CRVO. HRVO has a pathophysiology similar to that of CRVO but is managed similarly to BRVO. Prognosis is determined by the initial visual acuity and macular involvement. Less macular involvement results in a better visual acuity and a better prognosis.6 Only 20% of eyes are predisposed for an HRVO. To occur, veins draining the inferior and superior portions of the retina need to merge posterior to the lamina cribrosa and then form the central retinal vein. This allows occlusion at the lamina cribrosa of either the superior portion or the inferior portion of the central retinal vein, causing an HRVO.7
RISK FACTORS
Risk factors for all vein occlusions include hypertension, smoking, increased body mass index, hypercoagulable conditions, autoimmune and inflammatory conditions, diabetes, open-angle glaucoma, hyperlipidemia, oral contraceptives, and optic disc edema. Additional medical conditions may specifically lead to CRVO, including but not limited to syphilis, sarcoidosis, sickle cell disease, and HIV disease.4,5
OCULAR SIGNS OF VEIN OCCLUSIONS
Each type of retinal vein occlusion can be differentiated by the appearance of the retina on funduscopic examination.
A CRVO has an appearance that is commonly characterized as a “blood and thunder.” There will be multiple hemorrhages across the retina, cotton wool spots if there is ischemia, optic disc edema, and retinal nerve fiber layer damage (Figure 2). An HRVO has an appearance similar to that of a CRVO but respecting the horizontal raphe (Figure 3).6,7 As discussed above, nonischemic CRVO will have less-severe fundus findings described as hemorrhages in all quadrants, and vessel tortuosity but only mild dilation of the retinal vessels. Ischemic CRVO will appear as more severe hemorrhaging across the retina with retinal edema, and moderate to severe venous dilation with tortuosity and cotton wool spots are usually present, as well. If the CRVO is longstanding, neovascularization of the iris, optic disc, or retina may be present.4

Figure 2. Fundus photo of the right eye with CRVO showing flame hemorrhaging in all 4 quadrants of the posterior pole with optic disc edema.

Figure 3. Fundus photo of the left eye with inferior HRVO showing flame hemorrhaging across the lower half of the retina.
A BRVO has an appearance on fundus examination that is sectorial in nature and respecting the horizontal raphe (Figures 4A and 4B). Signs include superficial hemorrhages, cotton wool spots if there is ischemia, dilated tortuous veins, and potential retinal edema. If the BRVO is longstanding, neovascularization of the iris, optic disc, or retina may be seen but with less likelihood compared to CRVO. It is also important to note that in later stages of BRVO many signs can occur including, collateral shunt vessels, microaneurysms, hard exudates, and sclerosis of the veins.5

Figure 4A. Fundus photo of the left eye with supratemporal BRVO showing dot/blot and flame hemorrhages.

Figure 4B. Zoomed-out view of Figure 4A showing the BRVO located within the superior temporal portion of the retina.
It is important to note that all retinal vein occlusions can present certain additional risks to patients. Some of the major risks include neovascular glaucoma if neovascularization of the iris occurs, tractional retinal detachment from vitreous pulling on retinal neovascularization, and a vitreous hemorrhage if rupture of preretinal neovascularization occurs.4,5
MANAGEMENT
If a patient presents with sudden painless vision loss, evaluation with a detailed case history and fundus examination should be attempted with direct ophthalmoscopy. If the patient first presents to an optometrist or ophthalmologist, dilated fundus examination and optical coherence tomography (OCT) of the macula will be performed. If the macula shows edema on OCT (Figures 5A and 5B), referral to a retinal specialist is recommended. A retinal specialist will be able to administer intravitreal corticosteroid or initiate an anti–vascular endothelial growth factor (anti-VEGF) treatment regimen for the patient (Video). The patient usually continues to receive a series of anti-VEGF injections.4,8 Treatment with anti-VEGF is initiated early, especially if nonperfusion of the retina is present. Nonperfusion of the retina can be identified with traditional fluorescein angiography or OCT-angiography (Figure 6). Anti-VEGF treatment usually lasts for more than a year. If the patient remains stable for 6 months without anti-VEGF after the first year, they can be monitored every few months. If stability is not present, the patient may need additional anti-VEGF injections. Intravitreal corticosteroid can provide benefit in patients initially, but that benefit may regress. Long-term use of corticosteroids can cause complications; however, certain cases may require continuous intravitreal corticosteroids.8 HRVO is managed very similarly to CRVO.6

Figure 5A. OCT scan showing BRVO presenting with macular edema within the inner nuclear layer (arrow).
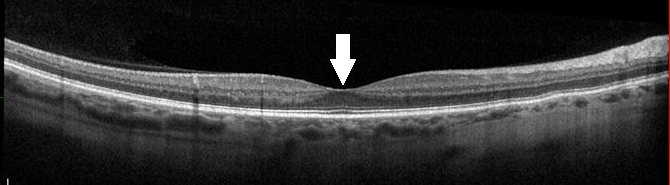
Figure 5B. Normal OCT scan of the macula. The arrow shows a normal appearance of the same area that has edema in Figure 5A.
Video. Intravitreal injection of anti-VEGF agent in a patient with a vein occlusion in the right eye.

Figure 6. OCT angiography of the left eye with a BRVO in the superior temporal quadrant. The dark areas are known as blockage and correspond to retinal hemorrhages. The superior retinal veins are dilated and show inflammation signifying leakage.
BRVO occlusions will also require a referral to a retinal specialist after initial evaluation. BRVO is often managed with both laser photocoagulation and anti-VEGF injections if nonperfusion is present. There is research that scatter laser photocoagulation reduces the chance of neovascularization developing in patients with a BRVO.5,8
The patients’ primary care physician needs to be involved in all of these cases and help manage the systemic risk factors, including hypertension, diabetes, hypercoagulable conditions, and other risk factors. Based on their risk factors, patients will need an assessment that may include fasting blood glucose level, hemoglobin A1c level, lipid profile, erythrocyte sedimentation rate, C-reactive protein level, syphilis testing, angiotensin-converting enzyme level, a complete blood cell count with differentials and platelets, and prothrombin time/partial thromboplastin time. In appropriate cases, a hypercoagulable panel (ie, protein C activity, protein S activity, homocysteine, antiphospholipid antibody, antithrombin III, factor V Leiden) may need to be obtained. Patients should also be educated on cessation of smoking and maintaining a healthy weight and lifestyle.5
CONCLUSION
Overall it is important that a patient with sudden unilateral painless vision loss be recognized and managed appropriately. Primary care physicians need to be aware that these cases need referral to a retinal specialist in order to properly manage the ocular condition. The primary care physician is also critical in managing the systemic factors that have contributed to a vein occlusion.
REFERENCES:
- Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133-143.
- Pichi F, Lim JI, Tripathy K, Gill MK, Shah VA. Central retinal vein occlusion. American Academy of Ophthalmology EyeWiki. Updated December 13, 2019. Accessed March 24, 2020. https://eyewiki.aao.org/Central_Retinal_Vein_Occlusion
- Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228(3):201-217. doi:10.1007/bf00920022
- Blair K, Czyz CN. Central retinal vein occlusion. StatPearls. Updated November 18, 2019. Accessed March 24, 2020. https://www.ncbi.nlm.nih.gov/books/NBK525985
- Cochran ML, Mahabadi N, Czyz CN. Branch retinal vein occlusion. StatPearls. Updated August 3, 2019. Accessed March 24, 2020. https://www.ncbi.nlm.nih.gov/books/NBK535370
- Yalamanchi S, Flynn HW Jr. Hemiretinal vein occlusion with macular hemorrhage and edema treated with intravitreal bevacizumab. Clin Ophthalmol. 2011;5:1509-1513. doi:10.2147/OPTH.S23698
- Buddi R, Eliott D. Evaluation and management of retinal vein occlusion. Rev Ophthalmol. November 15, 2004. Accessed March 24, 2020. https://www.reviewofophthalmology.com/article/evaluation-and-management-of-retinal-vein-occlusion
- Stuart A. Untangling retinal vein occlusion. EyeNet Magazine. November 2013. Accessed March 24, 2020. https://www.aao.org/eyenet/article/untangling-retinal-vein-occlusion


