Peer Reviewed
Pemphigus Vulgaris Successfully Treated With Rituximab
Authors:
Robert D. Field, MD
Marine Aviation Weapons and Tactics Squadron One, Marine Corps Air Station Yuma, Arizona
James J. Contestable, MD
Department of Dermatology Naval Medical Center San Diego, California
Disclaimer:
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government. All authors are military service members. This work was prepared as part of their official duties.
Citation:
Field RD, Contestable JJ. Pemphigus vulgaris successfully treated with rituximab. Consultant. 2019;59(4):108-110, 118.
A 44-year-old active-duty service member stationed in Japan presented to his primary care physician with a single shallow 1.5-cm nonhealing erosion on his scalp for 3 weeks (Figure 1). The erosion was cultured and grew high quantities of methicillin-resistant Staphylococcus aureus, and he initially was treated with a 14-day course of topical mupirocin and trimethoprim-sulfamethoxazole. This was followed by a 10-day course of clindamycin and topical gentamicin, neither of which provided benefit.

Figure 1. Scalp erosion at initial presentation.
Three months after his initial presentation, the initial lesion was followed by the development of flaccid bullae and erosions on the torso, nose, and scalp (Figures 2-4). Shortly thereafter, a hemorrhagic bulla appeared in the oral mucosal (Figure 5). The hands and feet were spared.
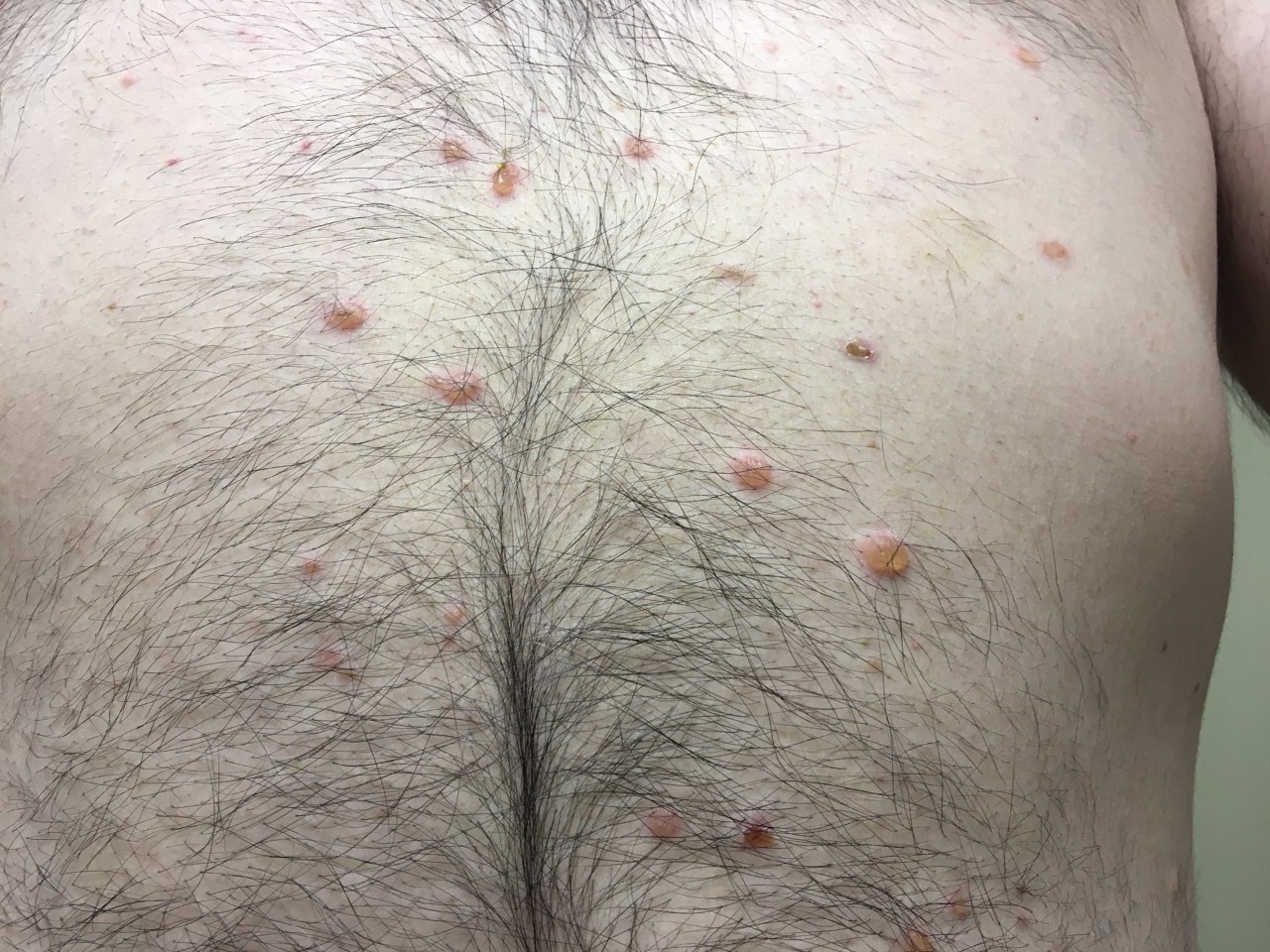
Figure 2. Torso prior to treatment with prednisone.

Figure 3. Left nasal ala erosion with hemorrhagic crust.

Figure 4. Vertex of the scalp with an erosive plaque.

Figure 5. Left buccal mucosa with hemorrhagic bullae.
An autoimmune blistering disorder was suspected, and he was referred to a local Japanese hospital where a dermatologist performed a biopsy and laboratory workup. He was initiated on oral prednisone, 25 mg every morning and 15 mg every evening; oral minocycline, 100 mg twice daily; and oral nicotinamide, 500 mg twice daily for suspected autoimmune bullous disorder. The biopsy showed positive direct immunofluorescence in a net-like pattern of the epidermis, and bloodwork was positive for elevated serum desmoglein 1 (Dsg1) and desmoglein 3 (Dsg3) antibodies, which confirmed the diagnosis of pemphigus vulgaris (PV).
After 2 weeks of treatment, his symptoms still were uncontrolled, and he was covered with 100 to 150 blisters over his face, trunk, neck, and scalp. The prednisone dose was then increased to 40 mg twice daily. After 2 weeks at this dose, his lesions began to heal, and new lesions became less frequent. Treatment with rituximab was discussed, but the medication was not available at military treatment facilities in Japan. Following a resource-based assessment, the patient and provider opted for a trial of azathioprine (AZA) and prednisone taper.
After 8 weeks of treatment with AZA, the patient’s liver function enzymes became elevated, with an aspartate aminotransferase level of 676 U/L and an alanine aminotransferase level of 260 U/L, and AZA was discontinued. With few other medication options available, prednisone had to be continued to prevent worsening of his condition.
He was then transferred to the United States, and a rituximab infusion was started following the rheumatoid arthritis protocol, with 1000 mg on day 1 and 1000 mg on day 15. He experienced minimal adverse effects from the infusion, noting only mild nausea, chills, and myalgias. Following the rituximab infusion, he began a corticosteroid taper and progressed well, noting only a few new lesions and pruritus. He also was prescribed topical clobetasol as needed for any new eruptions or pruritic areas of skin. Tapering the prednisone past 15 mg daily proved difficult initially due to the development of new lesions and pruritus; however, with a slower taper rate, we successfully discontinued corticosteroids without additional corticosteroid-sparing agents.
Twelve months after having started systemic corticosteroid treatment, he had developed osteonecrosis of the femoral heads bilaterally and had gained 42 pounds. His femoral head osteonecrosis is currently being managed medically, and he is free from clinical manifestations of PV 11 months after the rituximab infusions.
DISCUSSION
Epidemiology. PV has a mean age of onset in the sixth and seventh decades of life and is equally distributed among men and women.1 It is found all over the world, with an incidence ranging from 0.76 to 5 new cases per million per year; in Japan, the incidence of PV is 3.5 cases per million per year.1 The incidence is higher among persons of Ashkenazi Jewish ancestry compared with the general population, with an incidence of 16 to 32 new cases per million per year.2,3
Pathogenesis. Pemphigus vulgaris is an autoimmune blistering disease characterized by autoantibodies targeting the desmosomal cadherins Dsg1 and Dsg3.4 These autoantibodies circulate and bind to the desmogleins, disrupting cell adhesion between keratinocytes and creating intraepidermal blisters or acantholysis. It is believed that the autoantibodies accomplish this through mechanisms that include both steric hindrance and interference with cell signaling, leading to keratinocyte detachment and cell death.5,6 Patients with mucocutaneous PV are positive for both Dsg1 and Dsg3 antibodies; however, patients with mucosal dominant PV are positive for Dsg3 but negative for Dsg1 antibodies.
Clinical manifestations. In more than 50% of cases, patients with PV present with oral lesions that often precede cutaneous involvement by weeks or months.7 Other nonmucosal sites include the face, trunk, intertriginous areas, and scalp, whereas the palms and soles are usually spared. Lesions appear as flaccid blisters with a thin and fragile epidermal covering, varying in size from 1 cm to several centimeters. These blisters are easily ruptured, leaving behind erosions that are prone to bleeding and infection. Severe infections are potentially life threatening. The blisters can be painful, and oral lesions may lead to weight loss and decreased nutritional status.
Differential diagnosis. The differential diagnosis for PV includes pemphigus foliaceus, paraneoplastic pemphigus, drug-induced pemphigus, bullous pemphigoid, and other blistering disorders.
Prognosis. The mortality rate for untreated PV previously had been high, with death occurring in nearly all cases usually due to cutaneous infections. However, with the use of corticosteroids and adjuvant therapy, mortality rates are now below 10%.7,8
Management. Therapeutic management of PV traditionally has relied on the use of systemic corticosteroids, topical corticosteroids, and augmentation with corticosteroid-sparing immunosuppressant drugs including AZA, cyclophosphamide, mycophenolate mofetil, or intravenous immunoglobulin to reduce complications of long-term corticosteroid use.8-12 Rituximab, a monoclonal antibody directed at CD20 B-cell surface antigens, has been used in the treatment of PV since 2002 with favorable results.13-18
Previously, rituximab had been reserved for recalcitrant cases,19 but recent studies show that it can be effective as a first-line agent.20-23 Dosages used were 375 mg/m2 body surface area once weekly for 4 weeks (the lymphoma protocol) or 2 infusions of 1000 mg at 2 weeks apart (the rheumatoid arthritis protocol).24 In a 2015 review evaluating 578 patients, no difference in efficacy was noted between the lymphoma protocol and the rheumatoid arthritis protocol.25 At this time, there is no consensus about the preferred treatment protocol. Given the ease of administration, only requiring 2 visits, and decreased cost, the rheumatoid arthritis protocol was used for our patient. The side-effect profile is generally safe, although adverse events have been reported, including infections, septicemia, and death.26 Relapses occur in 40% to 60% of patients; however, this can be successfully treated with repeated rituximab infusions.20
Treatment of PV with long-term systemic corticosteroids is associated with many acute and long-term complications.27 The patient described here was no exception and developed osteonecrosis of the bilateral femoral heads as well as weight gain. Corticosteroid-sparing agents should be started as soon as practical to avoid these complications, and rituximab therapy should be strongly considered when possible.
Administration of first-line rituximab with short-term prednisone compared with long-term prednisone alone has been shown to have fewer severe adverse events and allowed for rapid corticosteroid tapering, reducing the likelihood of long-term corticosteroid-associated complications.21 Additionally, first-line rituximab led to an increase in the achievement of complete remission off-therapy after 2 years, and longer cumulative duration of remission off-therapy, which was 7-fold higher than with prednisone alone.21
Despite these benefits, rituximab is not without drawbacks. Infusions are expensive and can be time-consuming. Additionally, patients must be carefully monitored following treatments, because infusion reactions, while rare, can be severe. Administration may not be practical for patients in overseas and remote locations, such as this active-duty service member, due to the scarcity of resources and the difficulty of obtaining nonstandard laboratory tests.
The results of studies using rituximab as a first-line therapy in new-onset moderate to severe PV are promising. Current PV treatment guidelines vary by country and may not include first-line treatment with rituximab.28 Given the alternative of potential long-term systemic corticosteroids and/or corticosteroid-sparing agent, rituximab has many advantages.
- Amagai M. Pemphigus. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. Vol 4th ed. Philadelphia, PA: Elsevier; 2018:chap 29.
- Ahmed AR, Yunis EJ, Khatri K, et al. Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci U S A. 1990;87(19):7658-766
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29(4):432-436.
- Beutner EH, Jordan RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc Soc Exp Biol Med. 1964;117:505-510.
- Saito M, Stahley SN, Caughman CY, et al. Signaling dependent and independent mechanisms in pemphigus vulgaris blister formation. PLoS One. 2012;7(12):e50696.
- Grando SA. Pemphigus autoimmunity: hypotheses and realities. Autoimmunity. 2012;45(1):7-35.
- Pemphigus. In: Habif TP. Clinical dermatology : a color guide to diagnosis and therapy. 6th ed. Philadelphia, PA: Elsevier; 2016:64
- Bystryn J-C, Steinman NM. The adjuvant therapy of pemphigus: an update. Arch Dermatol. 1996;132(2):203-212.
- Hertl M, Zillikens D, Borradori L, et al. Recommendations for the use of rituximab (anti-CD20 antibody) in the treatment of autoimmune bullous skin diseases. J Dtsch Dermatol Ges. 2008;6(5):366-373.
- Goebeler M, Herzog S, Bröcker E-B, Zillikens D. Rapid response of treatment-resistant pemphigus foliaceus to the anti-CD20 antibody rituximab. Br J Dermatol. 2003;149(4):899-901.
- Kirtschig G, Murrell D, Wojnarowska F, Khumalo N. Interventions for mucous membrane pemphigoid/cicatricial pemphigoid and epidermolysis bullosa acquisita: a systematic literature review. Arch Dermatol. 2002;138(3):380-384.
- Hooten J, Hall R III, Cardones A. Updates on the management of autoimmune blistering diseases. Skin Therapy Lett. 2014;19(5):1-6.
- Daniel BS, Murrell DF, Joly P. Rituximab and its use in autoimmune bullous disorders. Dermatol Clin. 2011;29(4):571-575.
- Arin MJ, Engert A, Krieg T, Hunzelmann N. Anti-CD20 monoclonal antibody (rituximab) in the treatment of pemphigus. Br J Dermatol. 2005;153(3):620-625.
- Ahmed AR, Shetty S. A comprehensive analysis of treatment outcomes in patients with pemphigus vulgaris treated with rituximab. Autoimmun Rev. 2015;14(4):323-331.
- Joly P, D’Incan M, Musette P. Rituximab for pemphigus vulgaris. N Engl J Med. 2007;356(5):521; author reply 521-522.
- Cianchini G, Corona R, Frezzolini A, Ruffelli M, Didona B, Puddu P. Treatment of severe pemphigus with rituximab: report of 12 cases and a review of the literature. Arch Dermatol. 2007;143(8):1033-1038.
- Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128(12):2850-2858.
- Le Roux-Villet C, Prost-Squarcioni C, Alexandre M, et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol. 2011;147(7):843-849.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151(2):200-203.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031-2040.
- Craythorne EE, Mufti G, duVivier AW. Rituximab used as a first-line single agent in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2011;65(5):1064-1065.
- Cho Y-T, Lee F-Y, Chu C-Y, Wang L-F. First-line combination therapy with rituximab and corticosteroids is effective and safe for pemphigus. Acta Derm Venereol. 2014;94(4):472-473.
- Cholera M, Chainani-Wu N. Management of pemphigus vulgaris. Adv Ther. 2016;33(6):910-958.
- Wang H-H, Liu C-W, Li Y-C, Huang Y-C. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95(8):928-932.
- Ahmed AR, Shetty S. The emerging role of rituximab in autoimmune blistering diseases. Am J Clin Dermatol. 2015;16(3):167-177.
- Brown AE, Motaparthi K, Hsu S. Rituximab and intravenous immunoglobulin as alternatives to long-term systemic corticosteroids in the treatment of pemphigus: a single center case series of 63 patients. Dermatol Online J. 2018;23(12):7.
- Amagai M, Tanikawa A, Shimizu T, et al; Committee for Guidelines for the Management of Pemphigus Disease. Japanese guidelines for the management of pemphigus. J Dermatol. 2014;41(6):471-486.


