Peer Reviewed
Erlotinib-Induced Psoriasis
Authors:
Kory Schrom, MD
University Hospitals Cleveland Medical Center, Cleveland, Ohio
Maren Gaul, DO
Tanana Valley Clinic, Fairbanks, Alaska
Jonathan Bass, MD
MetroHealth Medical Center, Cleveland, Ohio
Citation:
Schrom K, Gaul M, Bass J. Erlotinib-induced psoriasis. Consultant. 2018;58(8):223-224.
A 90-year-old white woman with poorly differentiated squamous cell lung carcinoma presented for evaluation of a submammary and inguinal rash. The rash coincided with a recent increase of her erlotinib dosage from the dosage that she had been taking for the previous 3 months without dermatologic sequelae. The rash was associated with mild tenderness and had not responded to initial treatment with nystatin powder. The patient’s personal medical history and family medical history were negative for significant cutaneous disease, and findings of a review of systems were unremarkable.
Sharply marginated, nonscaling, submammary and inguinal plaques were noted bilaterally on physical examination. Similar lesions were noted on the right lateral neck and upper arms, with sparing of the intergluteal and perianal areas. The initial differential included a psoriasiform drug eruption vs intertrigo, with an id reaction.
The rash persisted with only central clearing despite treatment with desonide cream and ketoconazole cream for 2 weeks. In addition, involvement of flexural areas and nonintertriginous areas (ie, posterior neck, right forearm, and upper chest) along with pruritus developed. A biopsy of the right dorsal forearm and the left medial breast was obtained, and treatment was continued pending biopsy results.
The biopsies of both sites showed findings consistent with psoriasiform dermatitis and were both periodic acid–Schiff (PAS) negative (Figure).
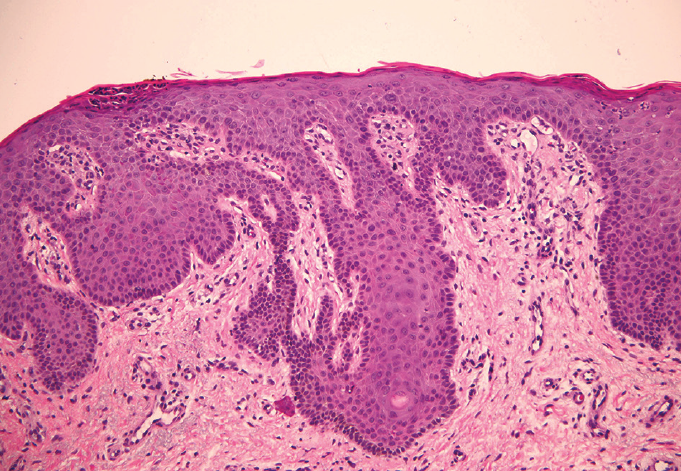
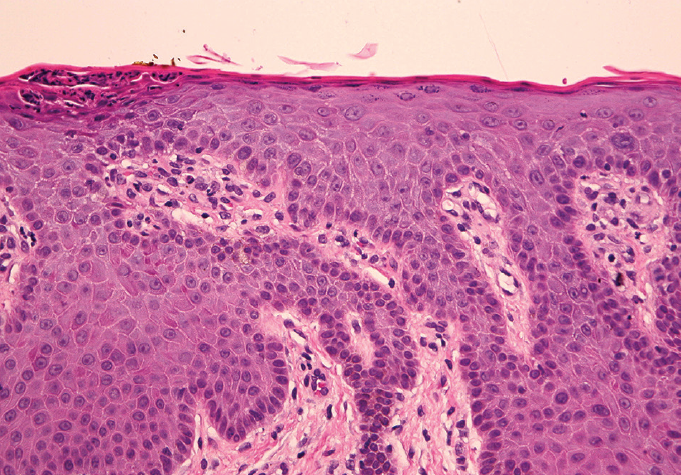
Figure: A punch biopsy specimen from the right forearm showing compact parakeratosis with intracorneal acute inflammation, psoriasiform epidermal hyperplasia with focal neutrophilic exocytosis, thinning of the suprapapillary plates, and mild chronic dermal inflammation (hematoxylin-eosin; original magnification Å~20 [left] and Å~40 [right]).
Ketoconazole was discontinued, and triamcinolone cream replaced the desonide cream on weekends. The patient was also advised to use over-the-counter second-generation H1 antihistamines for pruritus.
The patient’s oncologist was consulted to address the risks and benefits of erlotinib therapy cessation. Radiologic evidence supported the efficacy of erlotinib, so symptomatic relief of the rash was continued with modest response. The patient subsequently died from complications of cancer.
Discussion. Psoriasis is a multifactorial disorder with aberrant proliferative and immunologic pathways prominently contributing to its etiopathogenesis. Vital participants in the aberrant signaling of psoriasis are epidermal growth factor receptors (EGFRs), which are associated with the dysregulation of keratinocyte development and differentiation. Based upon the effect of the EGFR, it would be predicted that drugs used to slow this pathway would have a concomitant beneficial effect in patients with psoriasis. However, our patient developed de novo psoriasis while being treated with erlotinib for lung carcinoma.
Normal basal keratinocytes contain EGFRs that decrease with differentiation.1 Activation of EGFRs leads to receptor dimerization in the cell membrane and subsequent activation via autophosphorylation of intracellular tyrosine kinase domains; autophosphorylation is prevented by tyrosine kinase inhibitors (TKIs). Once the tyrosine kinase domains are activated, a downstream signaling pathway stimulates cell division, proliferation, and differentiation.2 In psoriatic lesions, there is a consistent increase in EGFRs, likely resulting from receptor persistence in sites of abnormal differentiation. As psoriatic lesions regress, the number of EGFRs decline in the epidermis.1
The 4 major skin toxic effects of TKIs, in order of decreasing incidence, are acneiform rash, pruritus, xerosis, and paronychia.3
The rash is seen more often with monoclonal antibodies rather than TKIs and is characterized by papules or pustules with a follicular distribution. Many medications have been reported to induce or exacerbate psoriasis, including TKIs, but erlotinib is not one of them.4 In fact, there have been at least 3 documented case reports in which patients treated with erlotinib saw improvement of their psoriasis.5,6
It is intriguing and paradoxical that EGFR inhibitors induce or exacerbate psoriasis, given their mechanism of action. Explanations might include a dose-dependent effect, synergy with concomitant drugs, or perhaps genomic subtypes of affected patients. Our observation of this paradoxical response potentially offers insights into the understanding of more-specific targets in the EGFR cascade and the potential for capturing their beneficial effects in the treatment of common dermatologic disease.
References:
- Nanney LB, Stoscheck CM, Magid M, King LE Jr. Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986;86(3):260-265
- Zorzou MP, Stratigos A, Efstathiou E, Bamias A. Exacerbation of psoriasis after treatment with an EGFR tyrosine kinase inhibitor. Acta Derm Venereol. 2004;84(4):308-309.
- Chen K-L, Lin C-C, Cho Y-T, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib, or afatinib treatment for non–small cell lung cancer. JAMA Dermatol. 2016;152(3):340-342.
- Milavec-Puretić V, Mance M, Ceović R, Lipozenćić J. Drug induced psoriasis. Acta Dermatovenerol Croat. 2011;19(1):39-42.
- Overbeck TR, Griesinger F. Two cases of psoriasis responding to erlotinib: time to revisiting inhibition of epidermal growth factor receptor in psoriasis therapy? Dermatology. 2012;225(2):179-182.
- Oyama N, Kaneko F, Togashi A, Yamamoto T. A case of rapid improvement of severe psoriasis during molecular-targeted therapy using an epidermal growth factor receptor tyrosine kinase inhibitor for metastatic lung adenocarcinoma. J Am Acad Dermatol. 2012;66(6):e251-e253.


