Peer Reviewed
Neuroleptic Malignant Syndrome: Overview of a Life-Threatening Medical Emergency
AUTHOR:
Tariq Sharman, MD, CMQ, CHCQM
Clinical Assistant Professor, Ohio University Heritage College of Osteopathic Medicine, Athens, Ohio; and Department of Medicine, Southern Ohio Medical Center, Portsmouth, Ohio
CITATION:
Sharman T. Neuroleptic malignant syndrome: overview of a life-threatening medical emergency. Consultant. 2020;50(2):35-44, 64. doi:10.25270/con.2020.02.00001
ABSTRACT: Antipsychotics and antiemetics are commonly prescribed medications across medical specialties. Antipsychotics are used to manage conditions such as psychosis, schizophrenia, bipolar disorder, depression, acute delirium, acute agitation, generalized anxiety disorder and Tourette syndrome. Antiemetics are used to manage nausea and vomiting, mainly in the setting of pregnancy and chemotherapy, motion sickness, gastroparesis and migraines. One of the fatal adverse effects of these medications is neuroleptic malignant syndrome (NMS), which carries high morbidity and mortality. This article identifies the common agents implicated in NMS, the pathophysiology of the syndrome, the clinical signs and symptoms, the risk factors, the laboratory and imaging findings, and treatment strategies.
KEYWORDS: Neuroleptic malignant syndrome, antipsychotics, antiemetics
Neuroleptic malignant syndrome (NMS) is a lethal medical emergency associated with the use of neuroleptic agents and antiemetics that is characterized by a typical clinical syndrome of hyperthermia, rigidity, mental status alteration, and dysautonomia. Delay and colleagues first described the syndrome in 1960 in patients treated with high-potency antipsychotics; at that time it was called akinetic hypertonic syndrome.1
Incidence rates for NMS range from 0.07% to 2.2% among patients taking neuroleptic medications.2 Mortality has decreased from the earliest reports of 76% in the 1960s and has been more recently estimated from 10% to 20%.3,4 NMS is more common in men than in women, possibly because of greater use of neuroleptics among men rather than a greater susceptibility.5
PATHOPHYSIOLOGY AND RISK FACTORS
NMS can occur as a result of changes in dopamine signaling. Two theories have been suggested. The first proposed mechanism by which antipsychotics and antiemetics cause NMS is that of dopamine D2 receptor antagonism. It is hypothesized that central D2 receptor blockade in the nigrostriatal pathways, hypothalamus, and spinal cord leads to increased tremor and muscle rigidity through the extrapyramidal pathways. Hypothalamic D2 receptor blockade results in a raised temperature set point and impairment of heat-dissipating mechanisms.6-8 Additionally, D2 receptor blockade might cause NMS by removing tonic inhibition from the sympathetic nervous system, which results in sympathoadrenal hyperactivity and dysregulation, leading to autonomic dysfunction manifesting as labile blood pressure, tachycardia, and tachypnea.9
The second proposed mechanism is reduced dopamine signaling resulting from sudden withdrawal of dopaminergic agents, or when the drug dosage is abruptly reduced in people taking dopaminergic drugs such as levodopa for Parkinson disease.10 At peripheral level, antipsychotic medications lead to increased calcium release from the sarcoplasmic reticulum, resulting in increased contractility, which can worsen the rigidity and hyperthermia and increase the chance of rhabdomyolysis.11
Genetics also plays a role in NMS, as demonstrated by cases of NMS occurring in identical twins as well as in a mother and 2 of her daughters.12 Genetic studies have confirmed the presence of a specific allele of the dopamine D2 receptor gene that is overrepresented in persons with NMS.13
Symptoms usually develop during the first 10 to 14 days of neuroleptic therapy initiation. However, the association of the syndrome with drug use is idiosyncratic. It is not a dose-dependent phenomenon, but prescribing high doses is a risk factor.14-17 Causative medications include antipsychotics and antiemetics, mainly high-potency first-generation antipsychotic agents such as fluphenazine and haloperidol. But it also can occur with use of low-potency antipsychotics such as chlorpromazine and second-generation antipsychotic drugs such as clozapine and olanzapine, as well as antiemetic drugs such as prochlorperazine, metoclopramide, and promethazine (Table).
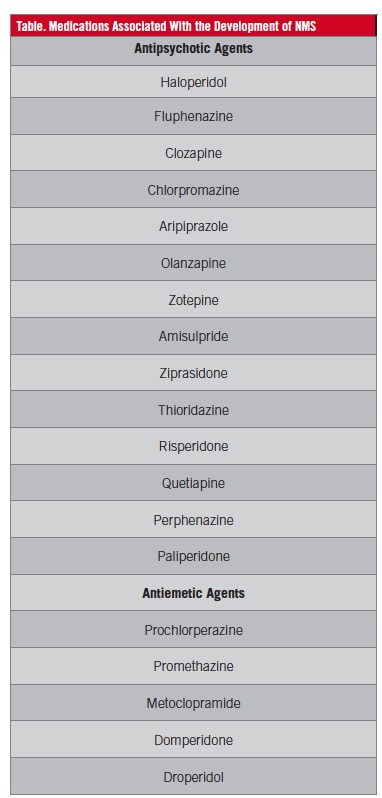
NMS can develop in the setting of withdrawal of l-dopa or dopamine agonist therapy, as well as with dose reductions and a switch from one agent to another in patients treated for Parkinsonism.10,18 Numerous risk factors have been implicated, including high-potency antipsychotics, rapid dose increase of antipsychotics, and using long-acting injectable neuroleptics such as fluphenazine enanthate and haloperidol decanoate.15,19 Other risk factors include dehydration, malnutrition, organic brain disease, and lithium usage.4,5,7,15,16
CLINICAL MANIFESTATIONS
NMS symptoms typically evolve over 1 to 3 days and include hyperthermia, rigidity, mental status changes, and autonomic dysregulation. Altered mental status occurs in the form of delirium with confusion and possible profound encephalopathy and coma.20 Muscular rigidity is mostly generalized and is associated with increased tone and described as “lead-pipe rigidity.” Rigidity can be accompanied with dystonia and dyskinesias.5,11 Hyperthermia with temperatures above 38°C are typical, but temperatures even greater than 40°C are not uncommon (up to 40% of cases).5 Autonomic dysregulation presents as tachycardia (88% of cases), labile or high blood pressure (up to 77% of cases), dysrhythmias, and tachypnea.21
NMS occurs within 10 days after the start of therapy in up to 90% of cases. The progression of symptoms is variable. However, in an analysis of 340 cases, 70% of patients followed a typical course of mental status changes appearing first, followed by rigidity, then hyperthermia and autonomic dysfunction.20 Physical examination will show signs and symptoms of autonomic instability, including diaphoresis, hyperthermia, tachycardia, tachypnea, hypoxemia, and labile blood pressure; signs and symptoms of decreased dopaminergic activity, including muscular rigidity, dystonia, and dyskinesia; and signs and symptoms of psychomotor agitation and altered mental status, including agitation, drowsiness, confusion, and coma.
Laboratory test abnormalities are nonspecific to the syndrome. A complete blood cell count will show leukocytosis and possibly thrombocytosis. Electrolyte abnormalities include hypocalcemia, hypomagnesemia, hyperkalemia, hyponatremia, hypernatremia, and metabolic acidosis. Elevated serum creatine kinase (CK) as high as 100,000 U/L may occur.7,20,21 CK elevation correlates with disease severity and prognosis.22 Myoglobinuric acute renal failure can result from rhabdomyolysis. Elevations of liver transaminases and lactic acid can be seen. A low serum iron concentration could be seen in patients with NMS and is a sensitive but not specific marker for NMS among acutely ill psychiatric patients.21,23 Other laboratory abnormalities include hyperuricemia and proteinuria.
EVALUATION AND DIAGNOSIS
A detailed history and physical examination are pivotal in the diagnosis of NMS in a patient who is taking a possible causative medication and who develops a typical clinical syndrome. Although no diagnostic test is available for NMS, testing has a fundamental role in the evaluation of patients with potential NMS. Typical laboratory abnormalities help to confirm the clinical diagnosis, some tests rule out other conditions, and still others are used to monitor patients for complications of NMS.
In patients with possible NMS, brain imaging studies and lumbar puncture are required to exclude structural brain disease and infection. Magnetic resonance imaging (MRI) and computed tomography (CT) findings typically are normal. Cerebrospinal fluid analysis results are usually normal, but a nonspecific elevation in protein can be reported. Electroencephalography can be done to rule out nonconvulsive status epilepticus. In patients with NMS, generalized slow-wave activity is seen.20,21,24
Due to the lack of generally accepted diagnostic criteria for NMS, an international multispecialty consensus group published diagnostic criteria for NMS in 2011.25 These criteria are based on positive clinical and laboratory findings as well as the exclusion of alternative causes, and each item is given a priority score for its relative importance in contributing to the diagnosis. These criteria are as follows: recent dopamine antagonist exposure or dopamine agonist withdrawal, hyperthermia, rigidity, mental status alteration, CK elevation, sympathetic nervous system lability, tachycardia plus tachypnea, and a negative workup for other causes.25
DIFFERENTIAL DIAGNOSIS
Other conditions can have signs and symptoms similar to NMS, including serotonin syndrome, malignant hyperthermia, lethal catatonia, cholinergic crisis, central nervous system infection, heat stroke, delirium tremens, and septic shock. A detailed medical history and a comprehensive physical examination, in addition to appropriate laboratory testing and imaging, can help narrow the differential diagnosis.
Serotonin syndrome is characterized by the triad of autonomic dysfunction, altered mental status, and movement disorder (tremor and abnormal involuntary movement) following exposure to a serotonergic agent. Laboratory findings characteristic of NMS (eg, elevated CK level, liver function test results, and white blood cell count) do not occur in serotonin syndrome. Serotonin syndrome can be distinguished from NMS in most cases by a detailed history of medication use and the presence of tremor and abnormal movements but the absence of severe rigidity. Treatment includes removal of the offending drug and supportive management.26-29
Lethal catatonia occurs in people with schizophrenia or during manic episodes. Neuroleptics might either improve or worsen the symptoms of lethal catatonia. Lethal catatonia tends to have a prodrome of excitement and agitation prior to the onset of rigidity, while NMS tends to begin with rigidity.30-32
Malignant hyperthermia is a genetic disorder due to an autosomal dominant mutation in the ryanodine receptor, which leads to excessive calcium release from the sarcoplasmic reticulum in skeletal muscles upon exposure to halogenated inhalational anesthetic agents and succinylcholine. It is usually distinguished from NMS by its clinical setting. Treatment is mainly supportive care and use of dantrolene to decrease calcium release, and subsequent avoidance of triggering medications.33-36
TREATMENT
Treatment consists of general measures that are mainly supportive and are directed at controlling rigidity and hyperthermia and preventing complications (eg, respiratory failure, renal failure, cardiac arrhythmias). The most important intervention is to discontinue all antipsychotics and to remove other potential contributing psychotropic agents (eg, serotonergic agents, lithium) if possible. Important interventions include maintaining cardiorespiratory stability, alkalinizing urine to help prevent acute renal failure due to rhabdomyolysis, and controlling fever using cooling blankets, gastric lavage, and ice packs, in addition to using benzodiazepines to control agitation.
Specific treatments include the use of dantrolene, bromocriptine, amantadine, and benzodiazepines. Dantrolene is a direct-acting skeletal-muscle relaxant and is effective in treating malignant hyperthermia (MH) by reducing heat production and rigidity. Dantrolene is hepatotoxic and should probably be avoided if liver function test results are very abnormal. While some recommend discontinuing dantrolene after a few days, others suggest continuing the drug for 10 days followed by a slow taper to minimize relapse. Based on an analysis of 271 case reports, the use of dantrolene in combination with other therapies (eg, bromocriptine) may be preferred over dantrolene alone due to lower mortality and longer complete time of remission.37-39 Bromocriptine is a dopamine agonist that helps restore lost dopaminergic tone. It is usually recommended to be continued for 10 days after NMS has been controlled and then tapered slowly. Common adverse effects include hypotension, headache, and dizziness. Amantadine has a dopaminergic effect and is used as an alternative to bromocriptine. Common adverse effects include orthostatic hypotension, syncope, peripheral edema, and hallucination. Benzodiazepine antispasmodic agents—mainly diazepam and lorazepam—are used to control agitation and rigidity.
A retrospective analysis of published cases of NMS indicated that the use of bromocriptine and/or dantrolene appeared to accelerate clinical response.40 In an animal model of NMS, dantrolene reduced body temperature, CK levels, and an electromyography activation measure of rigidity compared with controls.41 Recommendations for specific medical treatments in NMS are based on case reports and clinical experience. While evidence supporting the use of these agents is limited, they are frequently used because of anecdotal evidence of efficacy and the high morbidity and mortality of the disorder. A reasonable approach is to start with benzodiazepines (lorazepam or diazepam) along with dantrolene in moderate or severe cases, followed by the addition of bromocriptine or amantadine.42
ELECTROCONVULSIVE THERAPY
Electroconvulsive therapy (ECT) can help with the alteration of level of consciousness and temperature in patients with NMS. It may also be useful in treating the underlying psychiatric disease in patients who are unable to take neuroleptics.43 The rationale for the use of ECT in NMS includes its efficacy in treating malignant catatonia and improving parkinsonism. ECT with anesthesia is generally safe. However, serious complications such as cardiac arrest and ventricular fibrillation have been reported.43-46 ECT should be considered in patients who do not respond to medical therapy in the first week, those in whom residual catatonia persists after other symptoms have resolved, and those in whom lethal catatonia is suspected as an alternative or concomitant disorder.43,44 In a case series of 15 patients who had neurocognitive or schizophrenia spectrum disorders and who developed NMS after exposure to multiple antipsychotic drugs, all patients received bitemporal ECT after failed pharmacotherapy for NMS. ECT was well tolerated and resulted in a remission rate of 73.3%. Patients showed early initial response to ECT (mean of 4.2 treatments), but an average of 17.7 treatments was necessary to minimize recurrence of catatonic signs.47
PROGNOSIS
Most episodes of NMS resolve within 2 weeks.5,21 However, cases persisting for 6 months with residual catatonia and motor signs have been reported.48 The reported mortality rates for NMS vary from 5% to 20%. The occurrence of medical complications and disease severity are the strongest predictors of mortality.49,50
REFERENCES:
- Delay J, Pichot P, Lemperiere T, Elissalde B, Peigne F. A non-phenothiazine and non-reserpine major neuroleptic, haloperidol, in the treatment of psychoses [in French]. Ann Med Psychol (Paris). 1960;118(1):145-152.
- Gelenberg AJ, Bellinghausen B, Wojcik JD, Falk WE, Sachs GS. A prospective survey of neuroleptic malignant syndrome in a short-term psychiatric hospital. Am J Psychiatry. 1988;145(4):517-518. doi:10.1176/ajp.145.4.517
- Shalev A, Hermesh H, Munitz H. Mortality from neuroleptic malignant syndrome. J Clin Psychiatry. 1989;50(1):18-25.
- Modi S, Dharaiya D, Schultz L, Varelas P. Neuroleptic malignant syndrome: complications, outcomes, and mortality. Neurocrit Care. 2016;24(1):97-103. doi:10.1007/s12028-015-0162-5
- Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202. doi:10.1016/s0025-7125(16)30278-4
- Henderson VW, Wooten GF. Neuroleptic malignant syndrome: a pathogenetic role for dopamine receptor blockade? Neurology. 1981;31(2):132-137. doi:10.1212/wnl.31.2.132
- Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135. doi:10.1093/bja/85.1.129
- Gurrera RJ. Is neuroleptic malignant syndrome a neurogenic form of malignant hyperthermia? Clin Neuropharmacol. 2002;25(4):183-193. doi:10.1097/00002826-200207000-00001
- Jauss M, Krack P, Franz M, et al. Imaging of dopamine receptors with [123I]iodobenzamide single-photon emission-computed tomography in neuroleptic malignant syndrome. Mov Disord. 1996;11(6):726-728. doi:10.1002/mds.870110621
- Keyser DL, Rodnitzky RL. Neuroleptic malignant syndrome in Parkinson’s disease after withdrawal or alteration of dopaminergic therapy. Arch Intern Med. 1991;151(4):794-796. doi:1001/archinte.1991.00400040130031
- Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876. doi:10.1176/ajp.2007.164.6.870
- Otani K, Horiuchi M, Kondo T, Kaneko S, Fukushima Y. Is the predisposition to neuroleptic malignant syndrome genetically transmitted? Br J Psychiatry. 1991;158:850-853. doi:10.1192/bjp.158.6.850
- Mihara K, Kondo T, Suzuki A, et al. Relationship between functional dopamine D2 and D3 receptors gene polymorphisms and neuroleptic malignant syndrome. Am J Med Genet B Neuropsychiatr Genet. 2003;117B(1):57-60. doi:10.1002/ajmg.b.10025
- Pope HG Jr, Aizley HG, Keck PE Jr, McElroy SL. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
- Keck PE Jr, Pope HG Jr, Cohen BM, McElroy SL, Nierenberg AA. Risk factors for neuroleptic malignant syndrome. A case-control study. Arch Gen Psychiatry. 1989;46(10):914-918. doi:10.1001/archpsyc.1989.01810100056011
- Hermesh H, Aizenberg D, Weizman A, Lapidot M, Mayor C, Munitz H. Risk for definite neuroleptic malignant syndrome: a prospective study in 223 consecutive in-patients. Br J Psychiatry. 1992;161:254-257. doi:10.1192/bjp.161.2.254
- Berardi D, Amore M, Keck PE Jr, Troia M, Dell’Atti M. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome: a case-control study. Biol Psychiatry. 1998;44(8):748-754. doi:10.1016/s0006-3223(97)00530-1
- Wu Y-F, Kan Y-S, Yang C-H. Neuroleptic malignant syndrome associated with bromocriptine withdrawal in Parkinson’s disease—a case report. Gen Hosp Psychiatry. 2011;33(3):301.e7-301.e8. doi:10.1016/j.genhosppsych.2010.11.013
- Paparrigopoulos T, Tzavellas E, Ferentinos P, Mourikis I, Liappas J. Catatonia as a risk factor for the development of neuroleptic malignant syndrome: report of a case following treatment with clozapine. World J Biol Psychiatry. 2009;10(1):70-73. doi:10.1080/15622970701287369
- Velamoor VR, Norman RM, Caroff SN, Mann SC, Sullivan KA, Antelo RE. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173. doi:10.1097/00005053-199403000-00007
- Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146(6):717-725. doi:10.1176/ajp.146.6.717
- Hermesh H, Manor I, Shiloh R, et al. High serum creatinine kinase level: possible risk factor for neuroleptic malignant syndrome. J Clin Psychopharmacol. 2002;22(3):252-256. doi:10.1097/00004714-200206000-00004
- Lee JWY. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44(6):499-507. doi:10.1016/s0006-3223(98)00109-7
- Carbone JR. The neuroleptic malignant and serotonin syndromes. Emerg Med Clin North Am. 2000;18(2):317-325. doi:10.1016/s0733-8627(05)70127-9
- Gurrera RJ, Caroff SN, Cohen A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. 2011;72(9):1222-1228. doi:10.4088/JCP.10m06438
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120. doi:10.1056/NEJMra041867
- Birmes P, Coppin D, Schmitt L, Lauque D. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
- Bodner RA, Lynch T, Lewis L, Kahn D. Serotonin syndrome. Neurology. 1995;45(2):219-223. doi:10.1212/wnl.45.2.219
- Mason PJ, Morris VA, Balcezak TJ. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine (Baltimore). 2000;79(4):201-209. doi:10.1097/00005792-200007000-00001
- Fleischhacker WW, Unterweger B, Kane JM, Hinterhuber H. The neuroleptic malignant syndrome and its differentiation from lethal catatonia. Acta Psychiatr Scand. 1990;81(1):3-5. doi:10.1111/j.1600-0447.1990.tb06439.x
- Castillo E, Rubin RT, Holsboer-Trachsler E. Clinical differentiation between lethal catatonia and neuroleptic malignant syndrome. Am J Psychiatry. 1989;146(3):324-328. doi:10.1176/ajp.146.3.324
- Park J, Tan J, Krzeminski S, Hazeghazam M, Bandlamuri M, Carlson RW. Malignant catatonia warrants early psychiatric-critical care collaborative management: two cases and literature review. Case Rep Crit Care. 2017;2017:1951965. doi:10.1155/2017/1951965
- Caroff SN, Rosenberg H, Fletcher JE, Heiman-Patterson TD, Mann SC. Malignant hyperthermia susceptibility in neuroleptic malignant syndrome. Anesthesiology. 1987;67(1):20-25. doi:10.1097/00000542-198707000-00004
- Denborough M. Malignant hyperthermia. Lancet. 1998;352(9134):1131-1136. doi:10.1016/S0140-6736(98)03078-5
- MacLennan DH, Phillips MS. Malignant hyperthermia. Science. 1992;256(5058):789-794. doi:10.1126/science.1589759
- Wappler F. Malignant hyperthermia. Eur J Anaesthesiol. 2001;18(10):632-652. doi:10.1046/j.1365-2346.2001.00888.x
- Tsutsumi Y, Yamamoto K, Matsuura S, Hata S, Sakai M, Shirakura K. The treatment of neuroleptic malignant syndrome using dantrolene sodium. Psychiatry Clin Neurosci. 1998;52(4):433-438. doi:10.1046/j.1440-1819.1998.00416.x
- Bond WS. Detection and management of the neuroleptic malignant syndrome. Clin Pharm. 1984;3(3):302-307.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11(1):R4. doi:10.1186/cc5148
- Rosenberg MR, Green M. Neuroleptic malignant syndrome: review of response to therapy. Arch Intern Med. 1989;149(9):1927-1931. doi:10.1001/archinte.149.9.1927
- Tanii H, Taniguchi N, Niigawa H, et al. Development of an animal model for neuroleptic malignant syndrome: heat-exposed rabbits with haloperidol and atropine administration exhibit increased muscle activity, hyperthermia, and high serum creatine phosphokinase level. Brain Res. 1996;743(1-2):263-270. doi:10.1016/s0006-8993(96)01059-1
- Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981. doi:10.1177/1060028016657553
- Addonizio G, Susman VL. ECT as a treatment alternative for patients with symptoms of neuroleptic malignant syndrome. J Clin Psychiatry. 1987;48(3):102-105.
- Caroff SN, Mann SC, Keck PE Jr, Francis A. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol. 2000;20(2):257-259. doi:10.1097/00004714-200004000-00021
- Trollor JN, Sachdev PS. Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust N Z J Psychiatry. 1999;33(5):650-659. doi:10.1080/j.1440-1614.1999.00630.x
- Davis JM, Janicak PG, Sakkas P, Gilmore C, Wang Z. Electroconvulsive therapy in the treatment of the neuroleptic malignant syndrome. Convuls Ther. 1991;7(2):111-120.
- Morcos N, Rosinski A, Maixner DF. Electroconvulsive therapy for neuroleptic malignant syndrome: a case series. J ECT. 2019;35(4):225-230. doi:10.1097/YCT.0000000000000600
- Silva RR, Munoz DM, Alpert M, Perlmutter IR, Diaz J. Neuroleptic malignant syndrome in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(2):187-194. doi:10.1097/00004583-199902000-00018
- Tural U, Onder E. Clinical and pharmacologic risk factors for neuroleptic malignant syndrome and their association with death. Psychiatry Clin Neurosci. 2010;64(1):79-87. doi:10.1111/j.1440-1819.2009.02042.x
- Nakamura M, Yasunaga H, Miyata H, Shimada T, Horiguchi H, Matsuda S. Mortality of neuroleptic malignant syndrome induced by typical and atypical antipsychotic drugs: a propensity-matched analysis from the Japanese Diagnosis Procedure Combination database. J Clin Psychiatry. 2012;73(4):427-430. doi:10.4088/JCP.10m06791


