Peer Reviewed
Lichen Striatus
AUTHORS:
Nancy J. Landez, BA; Lisa de Ybarrondo, MD; and Lynnette Mazur, MD, MPH
McGovern Medical School, University of Texas Health Science Center, Houston, Texas
CITATION:
Landez NJ, de Ybarrondo L, Mazur L. Lichen striatus [published online November 25, 2019]. Consultant360.
A 22-month-old girl presented to the pediatric clinic with a 2-month history of a pruritic rash that had started on her chest and had spread down her left arm (Figures 1 and 2). She had no known sick contacts and had no change or contact with new detergents, lotions, soaps or other chemical substances. The mother applied hydrocortisone cream as needed for itching.
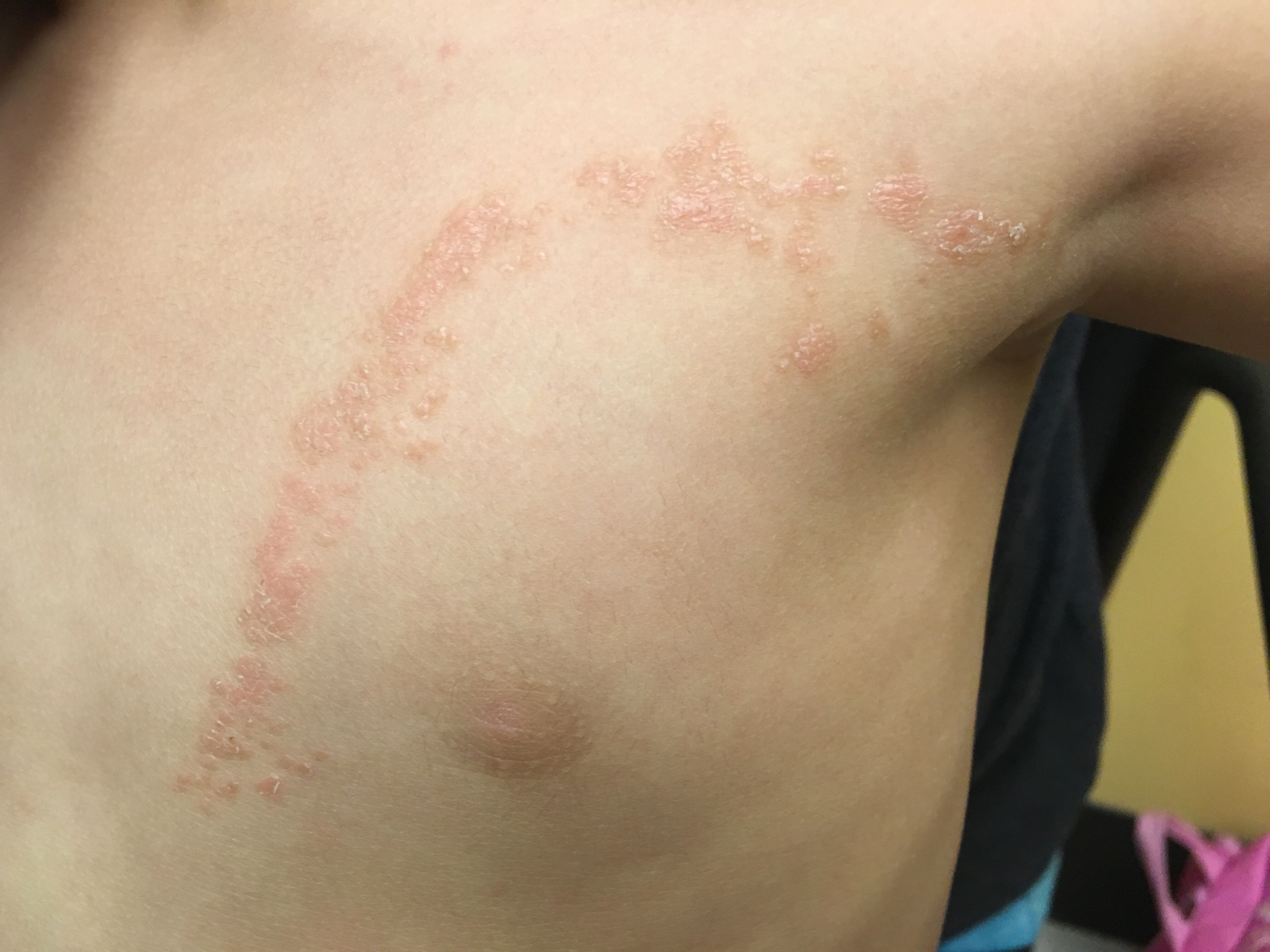
Figure 1. The initial presentation of the patient’s rash was confined to the left anterior chest wall in a curvilinear pattern.

Figure 2. Two months after the initial presentation, the rash had elongated down the patient’s left arm to the flexor of her elbow, continuing the U-shaped linear pattern on the chest wall.
Physical examination revealed linear, skin-colored, flat-topped papules that coalesced into an inverted U-shaped pattern along the upper left trunk and extended down the left arm in a linear pattern. There was no nail involvement. She was referred to a dermatologist and received a diagnosis of lichen striatus.
Discussion. Lichen striatus is a linear grouping of erythematous papules that occasionally have a vesicular component.1,2 Lesions range in color from pink, red, and tan to skin-colored and may be hypopigmented in dark-skinned individuals.2,3 Although the lesions are usually asymptomatic, pruritis occurs in 5% to 46% of patients and is more common in patients with atopy.4 The linear bands can be continuous or interrupted, 2 mm to 2 cm in width, and may involve the entire limb.2,5 The differential diagnosis includes genetic, infectious, and inflammatory causes (Table).
Table. Differential Diagnosis of Linear Dermatological Lesions | |||
Disease | Clinical Findings | Prognosis | Histopathology |
Lichen striatus | Unilateral asymptomatic grouping of inflammatory papules coalescing into linear arrangement following lines of Blaschko.1 Nail involvement confined to medial or lateral edges.6 | Regresses spontaneously within weeks to years.4,5 Postinflammatory hypopigmentation may persist for years.1,4,6 | Dense perivascular lichenoid lymphohistiocytic band surrounding necrotic keratinocytes and variable epidermal changes.5,6 |
Inflammatory linear verrucous epidermal nevus | Unilateral pruritic, erythematous, and hyperkeratotic papules that coalesce into plaques in a linear array.7 | Does not regress spontaneously, undergoes periods of exacerbation followed by improvement.4 | Chronic dermal inflammatory infiltrate, psoriasiform epidermal hyperplasia, alternating bands of orthokeratosis and parakeratosis.7 |
Linear lichen planus | Pruritic, polygonal, violaceous, flat-topped papules and plaques.6 Nail involvement involves multiple nails and the entire nail plate.8 Oral mucosal lesions often present.6 | Most cutaneous lesions spontaneously resolve within a few years.6 Often leaves a postinflammatory hyperpigmentation that takes years to fade.6 | Wedge-shaped hypergranulosis.6 Cytoid bodies at the dermoepidermal junction. Direct immunofluorescence positive for multiple IgM immunoglobins.8 |
Linear psoriasis | Well-demarcated, erythematous papules or plaques with overlying micaceous scales.9 | Patients may have other sequelae of psoriasis that determine the clinical course.9 | Epidermal hyperplasia, parakeratosis, neutrophils in the stratum corneum, thinned granular cell layer, tortuous dilated dermal papillary capillaries.10 |
Incontinentia pigmenti | Presents in neonatal period with linear papules and vesicles. Progresses to verrucous streaks in weeks to months, then hypopigmentation.11 | Permanent hyperpigmented whorls along lines of Blaschko. X-linked dominant, lethal in males.11 Often has other concurrent developmental abnormalities.12 | Eosinophilic spongiosis and intraepidermal vesicles and apoptotic keratinocytes in the epidermis. Marked melanin incontinence with numerous melanophages in the hyperpigmented stage.12 |
Linear Darier disease | Presents in teenage years with skin or yellow-brown keratotic papules on face, chest, and back.13 | Generally a chronic condition with frequent exacerbations from several external factors.13 | Acantholytic dyskeratosis.13 |
The 3 subsets of lichen striatus—typical lichen striatus, lichen striatus albus, and nail lichen striatus—are stratified by their morphologic variation.14 Typical lichen striatus is the most common type and occurs in 80% of cases. There is no hypopigmentation or nail involvement.14 Lichen striatus albus is more common in darker-skinned patients.2,15 Lesions in nail lichen striatus occur in isolation or in conjunction with skin lesions.2 These nail lesions may present with onychodystrophy restricted to a single nail and may lead to nail splitting, longitudinal ridging, and/or thinning.6,16
The lesions of lichen striatus are usually unilateral and most commonly occur on the limbs, followed by the trunk, head, and neck. They form along the lines of Blaschko, a system of lines representing the pathways of epidermal cell migration and proliferation during embryologic development of the skin.2,17 The lines are V-shaped on the posterior midline overlying the spine, S-shaped or whorled on the lateral and anterior abdomen, inverted U-shaped arcs on the chest, and perpendicular lines on the extremities.17,18 These unique shapes distinguish lines of Blaschko from neurologic dermatomes.18
The distinct distribution of lichen striatus lesions suggests cutaneous mosaicism. Genomic mosaicism is the result of postzygotic somatic mutations that produce abnormal keratinocyte clones that remain silent until triggered by an environmental event.17 The distribution of lines of Blaschko strongly supports this hypothesis, because the unique patterns are thought to be a result of the folding and stretching of an embryo during the first 2 months of organogenesis.19 Some suggest that it may be a functional mosaicism due to lyonization of the X chromosome.6 Regardless, it is classified as an acquired mosaic condition because it is not congenital and occurs when there is a break in normal immunologic function. An autoimmune response activates previously silent and invisible keratinocyte clones.3 Triggers may include viral infection, certain vaccinations, medications, and pregnancy.5 The increased incidence in patients with a history of atopy also strengthens the theory that an autoimmune response occurs in genetically susceptible individuals.2,4,5
The diagnosis is usually based on clinical findings. However, if a biopsy is done, the histologic examination reveals a dense lichenoid lymphohistiocytic band that is perivascular and surrounds the eccrine sweat glands and ducts.2,6 This inflammatory infiltrate is composed of CD3+ and CD8+ cells surrounding necrotic keratinocytes and activated Langerhans cells.6 Focal spongiosis of the epidermis, dyskeratotic changes that include epidermal hyperkeratosis and focal parakeratosis, and interface dermatitis are found in the overlying epidermis.3,6 These findings also support an autoimmune process.6,20
The onset of lichen striatus lesions can be sudden and progress over several weeks. The lesions regress spontaneously over 3 months to a year, but some resolve in as soon as 4 weeks while other cases have taken several years to resolve, especially cases with nail involvement.4,5 After resolution, postinflammatory hypopigmentation occurs in 25% to 50% of patients, more commonly in dark-skinned patients.1,4,6 Postinflammatory hyperpigmentation occurs in 3% to 33% of patients.4 Both can persist for months to years after resolution of the papular lesions.2,5
Given the condition’s self-limited course, therapy is not usually recommended. Patients can be counseled that the condition is benign, and that there are no long-term sequalae.2 Topical corticosteroids are useful if there is pruritis, but they do not hasten resolution.2,6 Topical calcineurin inhibitors, such as tacrolimus and pimecrolimus, may also be helpful for symptomatic patients.6 One report demonstrated that oral cyclosporine therapy led to complete regression of the rash in 4 weeks.3
Outcome of the case. We discussed the diagnosis and the benign nature of the disease with the girl’s mother. She was told that the rash could take 3 to 12 months to resolve, and that it did not require treatment. She was instructed to apply topical hydrocortisone to relieve the girl’s pruritus.
- Hauber K, Rose C, Bröcker EB, Hamm H. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10(7):536-539.
- Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51(4):606-624. doi:10.1016/j.jaad.2003.1012
- Romita P, Ettorre G, Bufano T, et al. Lichen striatus successfully treated with oral cyclosporine. Int J Immunopathol Pharmacol. 2017;30(4):420-422. doi:10.1177/0394632017744097
- Peramiquel L, Baselga E, Dalmau J, Roé E, del Mar Campos M, Alomar A. Lichen striatus: clinical and epidemiological review of 23 cases. Eur J Pediatr. 2006;165(4):267-269. doi:10.1007/s00431-005-0032-9
- Charifa A, Jamil RT, Ramphul K. Lichen striatus. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK507830/. Updated June 3, 2019. Accessed November 25, 2019.
- Payette MJ, Weston G, Humphrey S, Yu J, Holland KE. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33(6):631-643. doi:10.1016/j.clindermatol.2015.09.006
- Sugarman JL. Epidermal nevus syndromes. Semin Cutan Med Surg. 2007;26(4):221-230. doi:10.1016/j.sder.2008.03.006
- Kabbash C, Laude TA, Weinberg JM, Silverberg NB. Lichen planus in the lines of Blaschko. Pediatr Dermatol. 2002;19(6):541-545. doi:10.1046/j.1525-1470.2002.00229.x
- Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979-987. doi:10.1016/j.jaad.20007.029
- Weedon D. The psoriasiform reaction pattern. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. New York, NY: Churchill Livingstone Elsevier; 2010:chap 4.
- Swinney CC, Han DP, Karth PA. Incontinentia pigmenti: a comprehensive review and update. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):650-657. doi:10.3928/23258160-20150610-09
- Fusco F, Paciolla M, Conte MI, et al. Incontinentia pigmenti: report on data from 2000 to 2013. Orphanet J Rare Dis. 2014;9:93. doi:10.1186/1750-1172-9-93
- Meziane M, Chraibi R, Kihel N, Hassam B, Senouci K. Linear Darier disease. Dermatol Online J. 2008;14(12):11.
- Patrizi A, Neri I, Fiorentini C, Bonci A, Ricci G. Lichen striatus: clinical and laboratory features of 115 children. Pediatr Dermatol. 2004;21(3):197-204. doi:10.1111/j.0736-8046.2004.21302.x
- Paller AS, Mancini AJ. Eczematous eruptions in childhood. Paller AS, Mancini AJ, eds. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 5th ed. New York, NY: Elsevier; 2015:chap 3.
- Tosti A, Peluso AM, Misciali C, Cameli N. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6 pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Molho-Pessach V, Schaffer JV. Blaschko lines and other patterns of cutaneous mosaicism. Clin Dermatol. 2011;29(2):205-225. doi:10.1016/j.clindermatol.2010.09.012
- Happle R. Lyonization and the lines of Blaschko. Hum Genet. 1985;70(3):200-206. doi:10.1007/bf00273442
- Happle R. Mosaicism in human skin: understanding the patterns and mechanisms. Arch Dermatol. 1993;129(11):1460-1470. doi:10.1001/archderm.1993.01680320094012
- Jin H, Zhang G, Zhou Y, Chang C, Lu Q. Old lines tell new tales: Blaschko linear lupus erythematosis. Autoimmun Rev. 2016;15(4):291-306. doi:10.1016/j.autrev.2015.11.014


