Peer Reviewed
Aching and Burning Abdominal Pain in a 77-Year-Old Man
Authors:
Anthony Easterday, BS
Fourth-Year Medical Student, Creighton University School of Medicine, Omaha, Nebraska
Shailendra Saxena, MD, PhD
Department of Family Medicine, Creighton University School of Medicine, Omaha, Nebraska
Citation:
Easterday A, Saxena S. Duodenal adenocarcinoma [published online August 6, 2018]. Gastroenterology Consultant.
A 77-year-old man presented to an outpatient clinic with unspecified abdominal pain. The patient reported having had upper abdominal pain over a span of 5 to 7 days. He described the pain as aching and burning. During that period, he had become constipated, without the ability to pass flatus, and he had developed nausea. The patient also reported having had a decrease in appetite over the past 3 to 4 months. The patient did not describe any chest pain, shortness of breath, blood in the stool, or vomiting.
History. His relevant medical history included gastroesophageal reflux and the development of iron-deficiency anemia approximately 6 months prior to presentation, with a severe episode having occurred 2 months prior to presentation. The patient had never smoked, and he reported consuming alcohol occasionally. He was taking oral iron supplementation as a result of his anemic state.
During the anemic episode 2 months prior, his hemoglobin level was low at 7.1 g/dL, his mean corpuscular volume (MCV) was low at 61 µm3, his ferritin level was low at 5 ng/mL, his iron saturation was low at 4%, and his iron level was low at 16 µg/dL. The source of the anemia could not be localized despite his having undergone colonoscopy and upper endoscopy.
Physical examination. The patient was afebrile with stable vital signs at presentation. He appeared alert and uncomfortable due to the abdominal pain. Bowel sounds were decreased, with associated tenderness in the epigastric area. A high-pitched, hyperactive obstruction sound was appreciated upon auscultation. Abdominal examination findings were negative for hepatosplenomegaly and masses. There was no sign of rigidity, rebound tenderness, guarding, costovertebral angle tenderness, or McBurney point tenderness, and the Murphy sign was negative for gallbladder disease.
Diagnostic tests. At presentation, his hemoglobin had improved to 10.9 g/dL, his MCV to 67 µm3, his ferritin to 11 ng/mL, his iron saturation to 13%, and his iron level to 51 µg/dL. His amylase level was normal at 104 U/L. Results of liver function tests, a metabolic panel, a C-reactive protein test, and lipase tests were within normal limits.
Based on the history and physical examination findings, a diagnosis of intestinal obstruction was hypothesized. Abdominal radiography results displayed slightly dilated loops and stool within the intestines. Given the physical examination findings, the somewhat benign radiography results were insufficient to rule out obstruction. The patient agreed to further imaging despite the nonemergent appearance and past negative esophagogastroduodenoscopy (EGD) and colonoscopy findings.
Findings of computed tomography (CT) scans of the abdomen (Figure 1) showed that the third portion of the duodenum had collapsed. The CT results created a greater need for further workup for small bowel obstruction. EGD and colonoscopy are unable to reach the third portion of the duodenum; thus, capsule endoscopy was performed, and the results of barium studies (Figure 2) revealed a duodenal stricture in the third portion with extension to the proximal jejunum. Push enteroscopy revealed adenocarcinoma of the duodenum. The patient agreed to undergo surgical removal of the cancerous lesions.

Figure 1. A transverse view of the small bowel obstruction on the initial CT that was later determined to be duodenal adenocarcinoma.
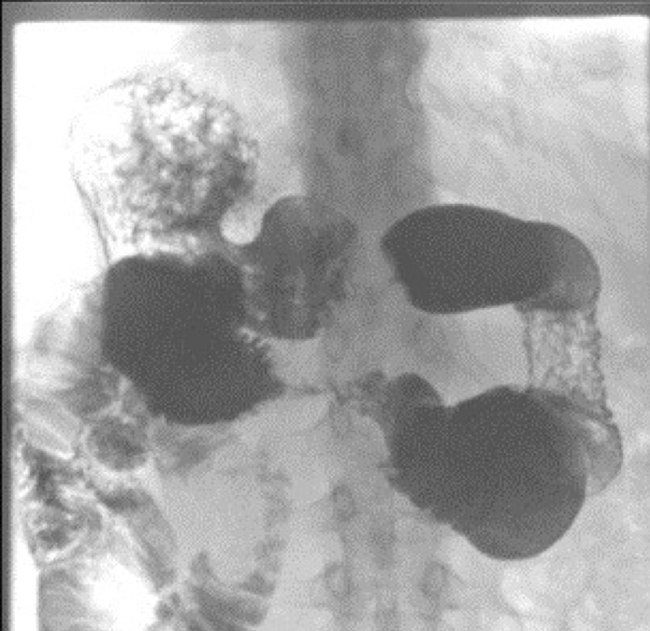
Figure 2. A view of the small bowel obstruction on the gastrointestinal series demonstrating an obstruction altering the flow of contrast in the duodenum.
The lesions were noted to be in the third and fourth portion of the duodenum and proximal jejunum, which were adhered to the posterior side of the superior mesenteric vasculature. The surgical intervention was an exploratory laparotomy with resection of the third and fourth portion of the duodenum and a hand-sewn duodenojejunostomy.
Pathology evaluation of the resected tissue suggested moderately differentiated adenocarcinoma of the duodenum with invasion through the muscle wall into the adjacent fibroadipose tissue, without spread to the biopsied lymph nodes. At a follow-up visit, the patient had been doing well, with some residual abdominal pain.
Discussion. The symptoms of anemia and abdominal pain are 2 separate occurrences that are encountered regularly in clinical settings. When they present together, diagnostic testing and imaging become much more important due to the potential presence of malignancy.
In the United States, cancer of the small intestine accounts for 0.6% of total cancer cases and 3.3% of gastrointestinal (GI) tract cancers.1 However, the incidence of all small bowel neoplasms has increased over time, with carcinoid and adenocarcinoma being the most common types diagnosed.2
The clinical challenge of finding the source of a bleed in an anemic patient is common in any clinic or hospital. A common cause of anemia is GI tract bleeding, which can be either upper or lower, depending on whether the bleed is proximal or distal to the ligament of Treitz. The bleeding can be described as either overt (melena or hematochezia with a found source), occult (iron-deficiency anemia with or without guaiac-positive stool with a found source), or obscure (no found source of bleeding).3
Concerning the duodenum and upper GI bleeding, it is recommended to first examine with an upper endoscopy, which can find the source of bleeding in 95% of cases.3 In our patient’s case, the upper endoscopy findings were negative. Concerning lower GI bleeding, it is recommended to perform nuclear scintigraphy in cases with active bleeding, colonoscopy in cases without active bleeding, and CT scan and capsule endoscopy if the bleeding is obscure.4 In our patient’s case, colonoscopy findings were negative for any source of bleeding, and his anemia improved as a result of iron replacement; however, her returned with abdominal pain and symptoms of bowel obstruction.
Acute abdominal pain due to intestinal obstruction is a common clinical presentation with a wide initial differential diagnosis. Our patient’s history of nausea, abdominal pain, a lack of flatus and defecation, and the physical examination findings of distention and high-pitched bowel sounds narrowed our differential diagnosis quickly. Small bowel obstruction can result in electrolyte and fluid imbalances, overgrowth of intestinal flora, compromised arterial perfusion and resultant ischemia, necrosis, and perforation. Common causes of obstruction include adhesions from prior surgical procedures, malignancy, and herniation. High-pitched bowel sounds on physical examination are more likely found in early obstruction compared with the minimal bowel sounds typical of late obstruction.5
In a patient presenting with suspected bowel obstruction, diagnostic imaging is indicated if the patient is clinically stable. Plain-film radiography has been shown to be the best initial method to identify small bowel obstruction, with CT being the best next step for localization and management planning.5,6 CT is the most sensitive and specific imaging modality for accurate evaluation of intestinal obstruction compared with ultrasonography and plain radiography.5,7 In our patient’s case, the plain film demonstrated dilated bowel loops, and CT found obstruction in the difficult-to-reach area of the third and fourth portion of the duodenum.
The modalities used to evaluate the small intestine include device-assisted enteroscopy (double-balloon enteroscopy, single-balloon enteroscopy, spiral enteroscopy, and balloon-guided endoscopy), push enteroscopy for proximal jejunal viewing, intraoperative enteroscopy, and capsule endoscopy. The least invasive of these is capsule enteroscopy. It is recommended that in cases of obscure GI bleeding capsule endoscopy be performed within 14 days.8
Concerning small bowel tumors, most patients have undergone EGD, colonoscopy, push enteroscopy, small bowel series, and CT scans prior to capsule endoscopy. One study found that the average workup before capsule endoscopy was 4.2 tests.9 This finding was accurate in our patient’s case. Moreover, 19% of tumors were missed with capsule endoscopy compared with 63% with other imaging modalities.9
This case was remarkable due to the diagnostic challenge involved with determining the source of anemia and obstructive symptoms. Such challenges can be addressed with a thorough physical examination at presentation and proper imaging modalities to determine the possible presence of common and rare disorders. The case demonstrates the need to trust physical examination findings and fully evaluate anemia and obstruction with additional imaging modalities such as CT and capsule endoscopy, despite negative findings on radiographs, colonoscopy, and EGD. Although rare, the potential miss of a lesion in the difficult-to-reach area of the third and fourth portion of the duodenum could be costly to the patient and his or her family.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63-71.
- Singh-Bhinder N, Kim DH, Holly BP, et al; Expert Panels on Vascular Imaging and Gastrointestinal Imaging. ACR Appropriateness Criteria®: nonvariceal upper gastrointestinal bleeding. J Am Coll Radiol. 2017;14(5 suppl):S177-S188.
- Darcy MD, Cash BD, Feig BW, et al; Expert Panel on Interventional Radiology. ACR Appropriateness Criteria®: radiologic management of lower gastrointestinal tract bleeding. American College of Radiology. https://acsearch.acr.org/docs/69457/Narrative/. Published 2006. Reviewed 201 Accessed July 25, 2018.
- Jackson PG, Raiji M. Evaluation and management of intestinal obstruction. Am Fam Physician. 2011;83(2):159-16
- Maglinte DD, Reyes BL, Harmon BH, et al. Reliability and role of plain film radiography and CT in the diagnosis of small-bowel obstruction. AJR Am J Roentgenol. 1996;167(6):1451-1455.
- Suri S, Gupta S, Sudhakar PJ, Venkataramu NK, Sood B, Wig JD. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40(4):422-428.
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2015;47(4):352-376.
- Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer. 2006;107(1):22-27.


