Peer Reviewed
Essential Thrombocytosis
Authors:
Simone Phang-Lyn, MS, OMS-IV
Dr. Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, Florida
Syed A. A. Rizvi, PhD, MS, MBA
Hampton University School of Pharmacy, Hampton, Virginia
Zafar Qureshi, MD
UHI CommunityCare Clinic, Miami, Florida
Sehrish Sikandar, MBBS
UHI CommunityCare Clinic, Miami, Florida
Citation:
Phang-Lyn S, Rizvi SAA, Qureshi Z, Sikandar S. Essential thrombocytosis. Consultant. 2019;59(9):286-287.
A 59-year-old woman with a history of hyperlipidemia and hypertension presented to clinic for follow up of asymptomatic essential thrombocytosis (ET).
Physical examination. On examination, the patient appeared healthy, well-nourished, and well-developed. Her height was 167 cm and her weight was 88 kg, with a corresponding body mass index of 31.2 kg/m2. She was slightly hypertensive, with a blood pressure in the left arm of 136/89 mm Hg while sitting, but all other vital signs were within normal limits. She was alert and oriented to person, place, and time. Findings of heart, lung, and abdominal examinations were unremarkable. Inspection of the skin revealed abnormal blood streaks within multiple nails (Figure).
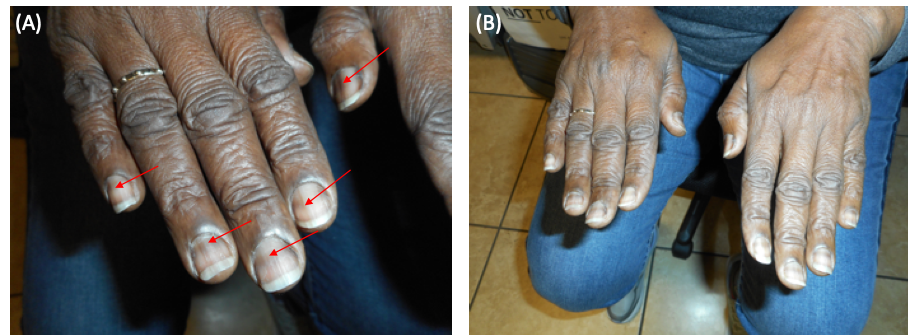
Figure. Melanonychia in the fingernails (red arrows).
Diagnostic tests. Laboratory tests results included the following values: white blood cell (WBC) count, 9600/µL (reference range, 3800-10,800/µL); red blood cell (RBC) count, 4.03 × 106/µL (reference range, 3.8-5.1 × 106/µL); hemoglobin, 13.5 g/dL (reference range, 11.7-15.5 g/dL); hematocrit, 39.5% (reference range 35.0%-45.0%); mean corpuscular volume, 98.0 µm3 (reference range, 80.0-100.0 µm3), mean corpuscular hemoglobin, 33.5 pg/cell (reference range, 27.0-33.0 pg/cell); red cell distribution width, 16.8% (reference range, 11.0%-15.0%); and platelet count, 1363 × 103/µL (reference range, 140-400 × 103/µL).
Discussion. ET is a rare myeloproliferative disorder with an incidence of 2 to 3 new cases per 100,000 population per year and normal life expectancy.1 It is characterized by findings of a persistently elevated platelet count above 450,000/µL, megakaryocyte hyperplasia, splenomegaly, and a clinical course complicated by thrombotic episodes, hemorrhagic episodes, or both. RBC counts, measured as hematocrit, are usually normal, while WBC counts can be normal or slightly elevated.2 Patients with ET have increased numbers of platelets due to sustained megakaryocyte proliferation and not due to prolonged platelet survival. Up to 90% of patients are found to have mutations in the janus kinase 2 gene (JAK2), the calreticulin gene (CALR), or the myeloproliferative leukemia virus oncogene (MPL); however, their role in pathogenesis is unknown.1
Most patients with ET are asymptomatic; nevertheless, they are encouraged to have regular platelet counts. A complete blood count every 3 months is recommended to monitor platelet levels in asymptomatic patients and those in steady state after treatment.3 Patients are risk-stratified for thrombotic complications through assessment of age, history of thrombosis, concurrent medical conditions and platelet count.4 High-risk patients include those who are 60 years of age or older and have a history of thrombosis or major bleeding but can also include patients with a platelet count of less than 1,500,000/µL.
The goal of therapy is to adequately control myeloproliferation and to keep platelet counts within normal range without toxicity or suppression of other marrow products.3 Treatment approaches include the use of aspirin, antiplatelet drugs, and myelosuppressive agents. First-line therapy of ET in high-risk patients usually includes hydroxyurea in conjunction with once-daily aspirin. Hydroxyurea, a cytoreducant, inhibits deoxynucleotide synthesis, which effectively reduces platelet counts.4-6
It is important to advise patients that treatment can help to control platelet counts and reduce the risk of thrombotic or hemorrhagic events. Patients should periodically follow up with a hematologist, as well as their primary care provider. In particular, patients requiring surgery or those who are or plan to become pregnant should consult their physician, because the medications used to treat ET may need to be stopped.
- Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood. 2016;128(20):2403-2414.
- Lal A. Essential thrombocytosis. Medscape. https://emedicine.medscape.com/article/206697-overview. Updated December 19, 2018. Accessed July 10, 2019.
- Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018;8(1):2.
- Harrison CN, Bareford D, Butt N, et al; British Committee for Standards in Haematology. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol. 2010;149(3):352-375.
- Tefferi A, Barbui T. Personalized management of essential thrombocythemia—application of recent evidence to clinical practice. Leukemia. 2013;27(8):1617-1620.
- Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132-113


